Darbepoetin alfa, injection, 200 micrograms/0.4 mL, 300 micrograms/0.6 mL and 500 micrograms/1 mL, Aranesp and Aranesp SureClick, November 2007
Public summary document for Darbepoetin alfa, injection, 200 micrograms/0.4 mL, 300 micrograms/0.6 mL and 500 micrograms/1 mL, Aranesp and Aranesp SureClick, November 2007
Page last updated: 29 February 2008
Public Summary
Document
Product: Darbepoetin alfa, injection, 200 micrograms/0.4 mL, 300 micrograms/0.6 mL
and 500 micrograms/1 mL, Aranesp and Aranesp SureClick
Sponsor: Amgen Australia Pty Ltd
Date of PBAC Consideration: November 2007
1. Purpose of Application
The submission sought to extend darbepoetin alfa’s current Section 100 (Highly Specialised
Drugs) PBS listing to include chemotherapy induced anaemia (CIA) in patients with
non-myeloid malignancies who meet certain criteria.
Highly Specialised Drugs are medicines for the treatment of chronic conditions, which,
because of their clinical use or other special features, are restricted to supply
to public and private hospitals having access to appropriate specialist facilities.
2. Background
At the June 2003 PBAC meeting, the PBAC rejected a submission seeking PBS-listing of darbepoetin for CIA on the basis of uncertain clinical benefit and resultant uncertain and unacceptable cost-effectiveness. The PBAC had previously considered changes in the need for transfusion and improvements in quality of life (QoL) and survival to be the patient-relevant outcomes for drugs that stimulate erythropoiesis. There was no evidence of any survival gain, and any QoL gain was very small and not statistically significant. The PBAC considered that the economic model should have been based on a more patient relevant outcome, preferably QoL.
3. Registration Status
TGA registration for the three darbepoetin alfa products proposed for listing commenced
on 7 June 2006. Darbepoetin alfa is indicated for the treatment of anaemia associated
with chronic renal failure (CRF). Darbepoetin alfa is also indicated for the treatment
of anaemia and reduction of transfusion requirements in patients with non-myeloid
malignancies where anaemia develops as a result of concomitantly administered chemotherapy.
4. Listing Requested and PBAC’s View
Private hospital authority required
Treatment of chemotherapy induced anaemia in patients with non-myeloid malignancies
who satisfy all of the following criteria:
- Haemoglobin level of less than 100 g per L [alternative option: 110 g per L];
- At risk of requiring transfusion;
- Scheduled to receive at least a further 12 weeks of chemotherapy;
- Adequate iron stores as defined by iron studies.
Treatment with darbepoetin alfa should be administered according to a fixed dose regimen
given every 3 weeks as described in the approved product information. The recommended
starting dose is 500 micrograms.
Therapy should be continued for approximately 4 weeks after the end of chemotherapy
or until haemoglobin concentrations approach 120 g per L.
A maximum of 6 packs per patient per course of chemotherapy will be PBS subsidised.
Note: Haemoglobin levels and rate of change should be monitored regularly and if required
the darbepoetin alfa dose adjusted or withheld according to the recommendations in
the approved product information.
If the clinical response of the patient (fatigue, haemoglobin response) is inadequate
after nine weeks, further therapy with darbepoetin alfa may not be effective.
This drug is not PBS-subsidised for treatment of anaemia in cancer patients who are
not receiving concurrent chemotherapy.
For PBAC’s view of the requested restriction, see Recommendation and Reasons.
5. Clinical Place for the Proposed Therapy
Anaemia is defined as a deficiency in the concentration of haemoglobin-containing
red blood cells (RBC) which deliver oxygen to the peripheral tissues to maintain the
viability of cells.
In Australia, CIA requiring intervention is currently managed by red blood cells transfusion.
Darbepoetin alfa has been shown to stimulate erythropoiesis in anaemic cancer patients,
resulting in the correction and maintenance of haemoglobin. Darbepoetin would provide
an alternative in the management of CIA.
6. Comparator
The submission nominated placebo or no pharmacological treatment as the comparator. This choice of comparator was previously accepted by the PBAC.
7. Clinical Trials
The submission presented seven new direct trials of darbepoetin alfa versus placebo:
- trials 114, 291(S1), 291 (S2), 232 and 145 are new placebo controlled trials, and
- trial 231 and trial 156 are new supplementary non- placebo controlled trials for the darbepoetin alfa 500g every three week regimen.
The submission also re-presented trials 297 and 161 that were included in the previous
submission.
The trials published at the time of submission were as follows:
|
Trial ID |
Protocol title/ Publication title |
Publication citation |
|---|---|---|
|
Direct randomised trial(s) |
||
|
Vansteenkiste J |
Double-blind, placebo-controlled, randomised Phase 3 trial of darbepoetin alfa in
lung cancer patients receiving chemotherapy. |
J Natl Cancer Inst 2002;94(16):1211-20. |
|
Hedenus M |
Efficacy and safety of darbepoetin alfa in anaemic patients with lymphoproliferative
malignancies: a randomised, double-blind, placebo-controlled study. |
British Journal of Haematology 2003;122:394-403. |
|
Hedenus M |
Randomised, dose-finding study of darbepoetin alfa in anaemic patients with lymphoproliferative malignancies. |
British Journal of Haematology 2002;119:79-86. |
|
Kotasek D |
Darbepoetin alfa administered every 3 weeks alleviates anaemia in patients with solid tumours receiving chemotherapy; results of a double-blind, placebo-controlled, randomised study. |
European Journal of Cancer 2003;39:2026-34. |
|
Supplementary randomised trials |
||
|
Canon J-L |
Randomised, double-blind, active-controlled trial of every-3-week darbepoetin alfa for the treatment of chemotherapy-induced anaemia, |
Journal of the National Cancer Institute 2006;98:273-84. |
- Several trials included patients whose anaemia was not predominantly due to chemotherapy;
- The inclusion criteria for most of the trials had a threshold for treatment of Hb 110g/L (different from proposed threshold of 100g/L).
- Most studies did not use the proposed dose of 500mcg every 3 weeks, and did not use the recommended duration of treatment. Studies 231 and 156 provide the only data for the proposed dose.
The endpoints used in the trials were highly variable. The definition of endpoints
was also variable and as such a post hoc analysis of patient-level data was performed
in the re-submission. It was agreed that the use of a post-hoc analysis was reasonable
in this instance.
8. Results of Trials
A primary outcome used in the economic evaluation in the submission was the proportion
of patients with transfusions from Week 1 to end of treatment period. The meta-analysis,
based on post-hoc analyses of data, was used for the economic evaluation.
The key results from the post hoc analyses are summarised in the tables below.
Results of the post-hoc analysis of transfusion incidence (week 1 to end of treatment
period) across the direct randomised trials
|
Trial ID |
Darbepoetin alfa |
Placebo |
Relative risk |
|---|---|---|---|
|
Trial 297 |
42/156(27%) |
82/158(52%) |
0.52 |
|
Trial 161 |
47/174(27%) |
70/170(41%) |
0.66 |
|
Trial 114 |
9/22(41%) |
6/11(55%) |
0.75 |
|
Trial 291 (S1) |
9/17(53%) |
23/51(45%) |
1.17 |
|
Trial 291 (S2) |
9/31(29%) |
12/31(39%) |
0.75 |
|
Trial 232 |
60/193(31%) |
85/193(44%) |
0.71 |
|
Pooled result (random effects) |
0.69 |
||
|
Chi-square for heterogeneity: 7.34 P=0.20; I2 statistic with 95% uncertainty interval = 31.9% |
|||
|
Trial 145 |
52/298(17%) |
116/298(39%) |
0.45 |
Bolded typography indicates statistically significant differences between treatment
groups
Transfusion incidence (week 1 to EOTP), meta-analysis of direct trials
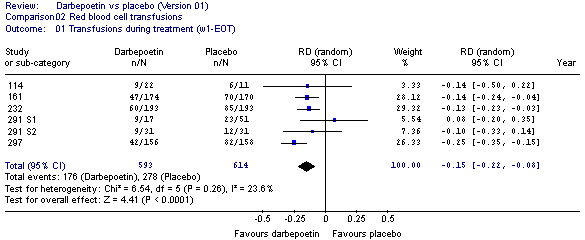
Results of the post-hoc analysis and the meta-analysis of the primary outcome, transfusion
incidence (week 1 to end of treatment period), across the direct randomised trials
showed that there was a significant reduction in the relative risk of receiving a
transfusion in the darbepoetin group.
The number of units transfused was very marginally significantly lower in the darbepoetin
versus the placebo group, and based primarily on the results of one trial (297). The
PBAC considered this outcome was likely to have had very minimal clinical significance.
Results of the post-hoc analysis of number of units transfused per patient across
the direct randomised trials
|
Trial ID |
Darbepoetin alfa |
Placebo |
Mean difference |
||
|---|---|---|---|---|---|
|
N |
mean (SD) |
N |
mean |
||
|
Trial 297 |
156 |
0.96(1.93) |
158 |
2.00(2.92) |
-1.04 (-1.59, -0.49) |
|
Trial 161 |
174 |
1.87(4.53) |
170 |
2.02(3.58) |
-0.15 (-1.01, 0.71) |
|
Trial 114 |
22 |
2.59(5.13) |
11 |
2.91(3.30) |
]-0.32 (-3.22, 2.58) |
|
Trial 291(S1) |
17 |
2.47(3.30) |
51 |
1.73(2.63) |
0.74 (-0.99, 2.47) |
|
Trial 292(S2) |
31 |
0.94(1.81) |
31 |
1.32(2.00) |
-0.38 (-1.33, 0.57) |
|
Trial 232 |
193 |
1.32(2.45) |
193 |
2.04(3.35) |
-0.72 (-1.31, -0.13) |
|
Pooled result (random effects) |
-0.60 (-0.99, -0.21) |
||||
|
Chi-square for heterogeneity: 6.15 P=0.29; I2 statistic with 95% uncertainty interval =18.7% |
|||||
Bolded typography indicates statistically significant differences
Another outcome used in the economic evaluation in the submission is change in haemoglobin
(Hb). A meta-analysis based on post-hoc analyses of data, was used for this economic
evaluation.
Results of the post-hoc analysis of change in Hb (baseline to end of treatment period)
across the direct randomised trials
|
Trial ID |
Darbepoetin alfa |
Placebo |
Mean difference |
||
|---|---|---|---|---|---|
|
N |
Mean (SD) |
N |
Mean(SD) |
||
|
Trial 297 |
155 |
1.06 (1.93) |
154 |
0 (1.52) |
1.06 ( 0.67, 1.45) |
|
Trial 161 |
171 |
1.99 (2.08) |
170 |
0.51 (1.34) |
1.48 ( 1.11, 1.85) |
|
Trial 114 |
22 |
1.77 (1.26) |
11 |
0.89 (0.98) |
0.88 ( 0.10, 1.66) |
|
Trial 291 (S1) |
17 |
1.23 (1.60) |
51 |
0.44 (1.42) |
0.79 (-0.06, 1.64) |
|
Trial 292 (S2) |
31 |
0.88 (1.50) |
30 |
0.33 (1.14) |
0.55 (-0.12, 1.22) |
|
Trial 232 |
192 |
1.25 (1.52) |
191 |
0.58 (1.39) |
0.67 ( 0.38, 0.96) |
|
Pooled result (random effects) |
0.94 (0.62, 1.27) |
||||
|
Chi-square for heterogeneity: 13.23 P=0.02;I2 statistic with 95% uncertainty interval =62.2% |
|||||
Bolded typography indicates statistically significant differences between treatment
groups
The PBAC considered that a mean difference of less than one g/dL (10 g/L) of Hb is
unlikely to be clinically significant.
A sub-group analysis of the population with baseline Hb < 100 g/L was performed to
apply the randomised comparative trial evidence to the requested restriction. In the
sub-group analysis, the incidence of transfusion during treatment was significantly
less in the darbepoetin group than in the placebo group, but the number of red blood
cell transfusions per transfused patient was not significantly different across the
two groups.
|
Hb change from baseline to end of treatment period |
Weighted mean difference (95% CI) |
|---|---|
|
Hb < 100 g/L |
0.93 (0.67, 1.19) |
As in the previous submission, there were no statistically significant improvements
in quality of life measurements related to fatigue.
The primary endpoint in trial 145 was survival. In this study, median survival time
(time to death) was 40 weeks for both darbepoetin and placebo patients, and the hazard
ratio was 0.93 (95% CI: 0.78, 1.11).
Study 231 was a parallel group, double-blind direct comparison of the proposed darbepoetin
dosing regimen with the weekly regimen (2.25mcg/kg) used in many of the other studies.
The primary endpoint was percentage of subjects with RBC transfusion from week 5 to
end of study. At baseline, 50% of the patients had Hb ≥ 100 g/L (above the proposed
threshold). Both efficacy and toxicity endpoints were not significantly different
between dosage regimens.
The re-submission also presented new toxicity data for the new trials. The key results
are summarised below.
Overview of adverse events in the direct trials
|
Trial ID |
Tx group |
Adverse Events |
Treatment-Related Adverse Events |
Withdraw due to AEs |
Deaths On-Trial |
|||
|---|---|---|---|---|---|---|---|---|
|
Severe |
Serious |
Any |
Severe |
Serious |
||||
|
Trial 297 |
DA |
69/155 (45%) |
60/155 (39%) |
13/155 |
4/155 (3%) |
1/155 (1%) |
10/155 (6%) |
22/155 (14%) |
|
PBO |
66/159 (42%) |
58/159 (36%) |
8/159 (5%) |
2/159 (1%) |
0/159 (0%) |
13/159 (8%) |
19/159 (12%) |
|
|
Trial 161 |
DA |
59/175 (34%) |
51/175 (29%) |
24/175 (14%) |
2/175 (1%) |
3/175 (2%) |
6/175 (3%) |
10/175 (6%) |
|
PBO |
59/169 (35%) |
63/169 (37%) |
17/169 (10%) |
3/169 (2%) |
3/169 (2%) |
7/169 (4%) |
4/169 |
|
|
Trial 114 |
DAa |
20/55 (36%) |
15/55 (27%) |
7/55 (13%) |
2/55 (4%) |
1/55 (2%) |
0/55 (0%) |
0/55 |
|
PBO |
6/11 |
6/11 |
0/11 (0%) |
0/11 (0%) |
0/11 (0%) |
0/11 (0%) |
0/11 |
|
|
Trial 291 (S1) |
DAa |
78/198 (39%) |
58/198 (29%) |
22/198 (11%) |
1/198 (1%) |
1/198 (1%) |
8/198 (4%) |
10/198 (5%) |
|
PBO |
19/51 (37%) |
18/51 (35%) |
6/51 (12%) |
0/51 (0%) |
0/51 (0%) |
0/51 (0%) |
4/51 |
|
|
Trial 291 (S2) |
DAa |
NR |
37/125 (30%) |
14/125 (11%) |
NR |
0/125 (0%) |
6/125 (5%) |
13/125 |
|
PBO |
NR |
7/31 |
3/31 |
NR |
0/31 (0%) |
2/31 (6%) |
0/31 |
|
|
Trial 232 |
DA |
102/194 |
84/194 (43%) |
13/194 |
3/194 (2%) |
3/194 (2%) |
11/194 (6%) |
17/194 (9%) |
|
PBO |
88/192 (46%) |
77/192 (40%) |
8/192 |
1/192 (1%) |
2/192 (1%) |
8/192 (4%) |
4/192 (10%) |
|
|
Trial 145 |
DA |
NR |
138 (46%) |
NR |
NR |
NR |
NR |
NR |
|
PBO |
NR |
121 (41%) |
NR |
NR |
NR |
NR |
NR |
|
a Darbepoetin alfa treatment groups combined.
DA = darbepoetin alfa; NR = not reported; PBO = placebo
Overview of adverse events in the supplementary trials
|
Trial 231 |
Trial 156 |
|||
|---|---|---|---|---|
|
DA 500g Q3w |
DA 2.25g/kg Qw |
DA 500g Q3w + spFe |
DA 500g Q3w + ivFe |
|
|
Subjects With at Least one On-study Adverse Event |
312/353 (88%) |
314/352 (89%) |
160/193 (83%) |
159/203 (78%) |
|
Treatment Related Adverse Events |
28/353 |
28/352 |
5/193 |
10/203 (5%)a |
|
Study Discontinuations Due to Adverse Events |
21/353 |
22/352 |
13/193 |
9/203 |
|
Fatal adverse events |
38/353 (11%) |
52/352 (15%) |
15/193 |
21/203 (10%) |
a Related to darbepoetin alfa, not iv iron.
DA = darbepoetin alfa. NR = not reported; ivFe = intravenous iron; spFe = standard
practice iron.
The extended assessment of comparative harms in relation to darbepoetin alfa was based
on the Oncologic Drugs Advisory Committee (ODAC) background document. Rigorous combined
analyses were performed to evaluate the safety of darbepoetin alfa, epoetin alfa,
and other ESAs in the chemotherapy-induced anaemia (CIA) population. Studies of patient
populations outside the registered CIA indication have caused concerns regarding tumour
progression, survival, and cardiovascular/thromboembolic events. The PBS listing requested
fits within the TGA approved use. However, the PBAC had some concerns that darbepoetin
would be used in patients with cancers where the use of ESAs in another clinical context
had an adverse effect on survival (e.g. head and neck cancer).
Pooled patient-level data analysis of darbepoetin alfa trials
Overall reported cardiovascular/thromboembolic (CV/TE) events occurred more frequently
in the darbepoetin alfa group compared to the placebo group (HR 1.15; 95% CI: 0.86,
1.52); this result was likely to have been heavily influenced by the subcategory of
TE events, which had a HR of 1.50 (95% CI: 0.97, 2.33). Hypertension was also reported
at a higher frequency in the darbepoetin alfa group compared to the placebo group
(HR 1.46; 95% CI: 0.78, 2.74). The HR for seizure was 1.43 (95% CI: 0.24, 8.58). None
of these hazard ratios were statistically significant.
There have been recent publications raising concern about risk associated with ESAs
in cancer, including a commentary in the NEJM that includes a summary of several studies
using darbepoetin in which treatment was associated with shorter overall survival.
However, these studies are in patient populations outside the registered CIA indication.
For PBAC’s view of these results, see Recommendation and Reasons.
9. Clinical Claim
The submission claimed that darbepoetin alfa is significantly more effective than
the main comparator (placebo/no pharmacological treatment) and has similar or less
toxicity.
For PBAC’s view of this claim, see Recommendation and Reasons.
10. Economic Analysis
The economic evaluation in the submission was based on results of a meta-analysis
of the direct randomised trials. The re-submission presented two economic models.
The first used treatment effects taken from the direct randomised trials, for the
population with baseline Hb < 110g/L. The second used a further analysis carried out
to determine the impact of applying the treatment effects to a subpopulation of patients
with baseline Hb < 100g/L. Results of the trials at endpoint (12 weeks) were used
in the economic analysis to provide effectiveness data at 16 weeks. The first model
was termed the ‘trial-based’ evaluation and the second the ‘modelled’ evaluation.
Drug costs were derived from the supplementary trials using the proposed dosage regimen
of darbepoetin alfa 500 g per 3 weeks. Costs of delivery of blood transfusion, blood
units, and thromboembolic events were modelled. Other costs related to the management
of chemotherapy-induced anaemia were excluded. The PBAC considered that the submission
overestimated the cost of transfusion.
A trial-based incremental cost/extra patient free of transfusion value in the range
$15,000 to $45,000 was calculated in the submission.
A trial-based incremental cost/extra transfusion avoided value of <$15,000 was also
calculated.
A base case modelled incremental cost/extra patient free of transfusion value in the
range $15,000 to $45,000 was calculated in the submission.
A base case modelled incremental cost/extra transfusion avoided value of <$15,000
was calculated.
In the majority of sensitivity analyses, the cost per transfusion avoided remained
< $15,000. These incremental cost effectiveness ratios were most sensitive to the
clinical results for the transfusion incidence and could be in the range of $15,000
- $45,000 (for the population with Hb < 110 g/L) and $105,000 - $200,000 (for the
subgroup with Hb < 100 g/L).
For further information on PBAC’s view of these analyses, see Recommendations and
Reasons.
11. Estimated PBS Usage and Financial Implications
The likely number of patients/year was estimated to be in the range 10,000 – 50,000
in Year 5. The financial cost/year to the PBS was estimated to be in the range $60M
- $100 M in Year 5.
The PBAC had significant uncertainty about the total cost to government and the estimates
provided by the submission.
12. Recommendation and Reasons
The PBAC considered that there were a number of issues that would need to be addressed
regarding the restriction and, in particular, the haemoglobin level at which therapy
should commence which should be consistent with the NHMRC and the Australian and NZ
guidelines for transfusion. Further, that ‘at risk of requiring transfusion’ and ‘adequate
iron stores’ should be more clearly defined. In the latter case, the PBAC was concerned
about possible use in patients with types of cancer where anaemia is known to occur
due to blood loss (e.g. colorectal cancer). However, these issues were not considered
to be a reason for rejection of the submission.
The PBAC noted the expert comment that there was insufficient evidence to conclude
that erythropoeisis-stimulating agents (ESAs), such as darbepoetin caused increased
harms or deaths in cancer patients due to stimulation of tumour growth, although there
is an increased incidence of thromboembolic events. The PBAC noted the extended assessment
of comparative harms in relation to darbepoetin alfa had been based on the Oncologic
Drugs Advisory Committee (ODAC) background document in the submission. Rigorous combined
analyses were performed to evaluate the safety of darbepoetin alfa, epoetin alfa,
and other ESAs in the chemotherapy-induced anaemia (CIA) population. Studies of patient
populations outside the registered CIA indication have caused concerns regarding tumour
progression, survival, and cardiovascular/thromboembolic events. The PBS listing requested
fits within the TGA approved use. However, the PBAC had some concerns that darbepoetin
would be used in patients with cancers where the use of erythropoeisis-stimulating
agents in another clinical context had an adverse effect on survival (e.g. head and
neck cancer).
The PBAC has previously considered changes in the need for transfusion and improvements
in quality of life (QoL) and survival to be the patient-relevant outcomes for drugs
that stimulate erythropoiesis. The PBAC noted that the trial data presented in the
submission showed no statistically significant improvement in QoL or survival compared
with blood transfusion, but show a statistically significant reduction in the proportion
of patients requiring transfusion, and the number of units transfused per patient.
In regard to safety, it was also noted that although there may be long term risks
associated with blood transfusion, these may be of a lesser concern to patients, whose
life expectancy is shortened due to their cancer, than improvement in quality of life.
While the clinical data clearly demonstrated that darbepoetin reduces chemotherapy-induced
anaemia, the proportion of patients requiring any blood transfusion, and could reduce
national demand for red blood cell units by a modest percentage, in the absence of
a survival benefit or significant improvement in quality of life for patients, the
base-case incremental cost-effectiveness ratios were considered unacceptably high.
The PBAC therefore rejected the submission because of uncertain cost-effectiveness.
13. Context for Decision
The PBAC helps decide whether and, if so, how medicines should be subsidised in Australia. It considers submissions in this context. A PBAC decision not to recommend listing or not to recommend changing a listing does not represent a final PBAC view about the merits of the medicine. A company can resubmit to the PBAC or seek independent review of the PBAC decision.
14. Sponsor’s Comment
Although disagreeing with the recommendation, the Sponsor intends to work collaboratively with the PBAC to find a way to move forward with reimbursement of darbepoetin alfa for the treatment of chemotherapy induced anaemia.



