Cinacalcet hydrochloride, tablets, 30 mg, 60 mg and 90 mg, Sensipar®
Public summary document for Cinacalcet hydrochloride, tablets, 30 mg, 60 mg and 90 mg, Sensipar®
Page last updated: 13 November 2009
Public Summary Document
Product: Cinacalcet hydrochloride, tablets, 30 mg,
60 mg and 90 mg, Sensipar®
Sponsor: Amgen Australia Pty Ltd
Date of PBAC Consideration: July 2009
1. Purpose of Application
The submission requested an extension to the current Section 85
Authority required listing to include use in:
patients with primary hyperparathyroidism in whom surgery is not a
treatment option; and
patients with persistent or recurrent hypercalcaemia following
resection of parathyroid carcinoma.
2. Background
A submission seeking PBS listing for the treatment of secondary
hyperparathyroidism in patients with end stage renal disease
receiving dialysis was rejected at the November 2005 PBAC meeting,
and again at the July 2006 PBAC meeting, because of uncertain
extent of clinical benefit and the resultant uncertain
cost-effectiveness.
At its November 2007 meeting, the PBAC recommended the listing of
cinacalcet on the PBS as a Section 100 (Highly Specialised Drug)
for the treatment for up to 6 months, by a nephrologist, of
patients with chronic kidney disease on dialysis who have sustained
secondary hyperparathyroidism, not responding to conventional
therapy on the basis of acceptable cost-effectiveness compared with
placebo. The PBAC agreed that cinacalcet should also be listed in
Section 85 for maintenance treatment as suggested by the Australian
and New Zealand Society of Nephrology, to enable patients in rural
areas easier access to continuing treatment.
3. Registration Status
Cinacalcet was registered by the TGA on 29 June 2005 for the following indications:
Treatment of the biochemical manifestations of secondary hyperparathyroidism in patients with end stage renal disease, receiving dialysis. It should be used as adjunctive therapy;
Treatment of hypercalcemia in patients with parathyroid carcinoma;
Treatment of the biochemical manifestations of primary hyperparathyroidism in patients for whom parathyroidectomy is not a treatment option.
4. Listing Requested and PBAC’s View
PRIMARY HYPERPARATHYROIDISM
Authority Required
Initiation and stabilisation, by a specialist, of a patient with
primary hyperparathyroidism for whom parathyroidectomy is not a
treatment option due to:
(i) previous parathyroidectomy failure, with non-localisable or
surgically inaccessible disease; OR
(ii) surgery representing excessive medical/surgical risk.
Authority Required
Maintenance therapy, following initiation and stabilisation of
treatment with cinacalcet, of a patient with primary
hyperparathyroidism who has after 6 months of treatment
demonstrated a reduction in serum calcium of ≥ 0.125
mmol/L.
NOTE:
During the titration phase, serum calcium should be monitored 4
weekly and the dose of cinacalcet titrated until an appropriate
serum calcium level is achieved. Approval will be limited to
sufficient quantity for 4 weeks treatment up to a maximum of 6
months supply with doses of up to 360 mg per day according to
patient’s response and tolerability.
During the maintenance phase, approval will be limited to provide
sufficient quantity for 4 weeks treatment up to a maximum of 6
months supply with doses up to 360 mg per day according to the
patient’s response and tolerability. During the maintenance
phase, serum calcium should be monitored 6 monthly.
Authority Required
Maintenance therapy for patients with primary hyperparathyroidism
who were receiving treatment with cinacalcet prior to [effective
PBS listing date]
PARATHYROID CARCINOMA
Authority Required
Initiation and stabilisation, by a specialist, of a patient with
persistent or recurrent hypercalcaemia following resection of
parathyroid carcinoma.
Authority Required
Maintenance therapy, following initiation and stabilisation of
treatment with cinacalcet, of a patient with parathyroid carcinoma
who has after 6 months of treatment demonstrated a reduction in
serum calcium of ≥ 0.125 mmol/L.
NOTE:
During the titration phase, serum calcium should be monitored 4
weekly and the dose of cinacalcet titrated until an appropriate
serum calcium level is achieved. Approval will be limited to
sufficient quantity for 4 weeks treatment up to a maximum of 6
months supply with doses of up to 360 mg per day according to
patient’s response and tolerability.
During the maintenance phase, approval will be limited to provide
sufficient quantity for 4 weeks treatment up to a maximum of 6
months supply with doses up to 360 mg per day according to the
patient’s response and tolerability. During the maintenance
phase, serum calcium should be monitored 6 monthly.
Authority Required
Maintenance therapy for patients with parathyroid carcinoma who
were receiving treatment with cinacalcet prior to [effective PBS
listing date]
The PBAC noted the Pre-PBAC response had revised the requested
listing for use in primary hyperparathyroidism (PHPT) to restrict
usage to moderate to severe cases defined as two consecutive
readings of serum calcium of > 2.85 mmol/L, and therefore the
Committee considered Study 204 most relevant in support of listing
for this patient group. The Pre-PBAC Response also proposed that a
response to treatment be defined as a reduction in serum > 0.25
mmol/L rather than > 0.125 mmol/L, as proposed in the submission
to address concerns raised by the ESC about appreciable measurement
error.
5. Clinical Place for the Proposed Therapy
Primary hyperparathyroidism (PHPT) refers to the inappropriate or
unregulated overproduction of PTH leading to an abnormal calcium
homeostasis, with no antecedent cause. Primary parathyroid
carcinoma is a rare cause of PHPT, accounting for less than 1% of
PHPT cases. Short term consequences of PHPT manifest through
effects on the renal, gastrointestinal, musculoskeletal,
neuropsychological and cardiovascular systems. Long term
consequences include an increased risk of cardiovascular events,
bone fractures and increased mortality. In patients with PT Ca, the
impact of the disease is such that patients with untreated or
incurable carcinoma will commonly die from the deleterious effects
of hypercalcaemia. Parathyroidectomy is the first treatment option
for patients with primary hyperparathyroidism and parathyroid
cancer. For patients in whom parathyroidectomy is not a viable
treatment option or who have had a parathyroidectomy failure there
are limited treatment options. The submission stated that
cinacalcet may provide a treatment option for such patients.
6. Comparator
The submission nominated placebo and standard medical management as
the main comparator. Standard medical management comprises of
specialist care, frusemide, saline, and sometimes supplemented by
therapy with vitamin D and bisphosphonates.
7. Clinical Trials
The submission presented one randomised trial, Trial 120, of cinacalcet versus placebo
in patients with mild-moderate PHPT (PTH greater than 4.74 mmol/L and serum calcium
between 2.58 and 3.10 mmol/L at baseline). The submission also presented two uncontrolled
studies - Study 159, an extension study of Trial 120, and Study 204 which enrolled
patients with PT Ca or with intractable PHPT with a serum calcium greater than 3.10
mmol/L at baseline.
The studies published at the time of submission are as follows:
| Trial ID/First Author | Protocol title/Publication title | Publication citation |
|---|---|---|
| Direct randomised trials | ||
| Trial 120/ Peacock, 2005 | Cinacalcet hydrochloride maintains long-term normocalcemia in patients with primary hyperparathyroidism | J Clin Endocrin Metab 2005; Vol 90(1):pages 135-141 |
| Non-randomised studies | ||
| Study 159/ Peacock, 2003 | Long-term control of primary hyperparathyroidism with cinacalcet HCl (AMG 073) | J Bone Miner Res 2003; Vol 18(suppl 2):S17, Abstract 1060 |
| Study 204 Silverberg, 2007 Peacock, 2002 Rubin, 2003 Silverberg, 2003 Peacock, 2004 Rubin, 2004 Silverberg et al:, 2004 | Cinacalcet reduces serum calcium in inoperable parathyroid carcinoma Normalization of hypercalcaemia with calcimimetic AMG 073 in a patient with metastatic parathyroid cancer. Effective management of severe hypercalcaemia with calcimimetic cinacalcet HCl in patients with PT Ca The effects of cinacalcet on serum calcium in patients with PT Ca or recurrent primary HPT after parathyroidectomy Cinacalcet HCl is an effective therapy for hypercalcaemia of primary hyperparathyroidism across a broad range of patients Clinical course of 10 patients with inoperable PT Ca treated with calcimimetic cinacalcet HCl Cinacalcet HCl effectively treats hypercalcaemia in patients with PT Ca | J Clin Endocrin 2007; Vol 92(10):3803-3808 J Bone Miner Res 2002; Vol 17(suppl 1):381, abstract SU392 J Bone Miner Res 2003; Vol 18(suppl 2):S393, abstract M410 J Bone Miner Res 2003; Vol 18(suppl 2):S171, abstract SA420 J Bone Miner Res 2004; Vol 19(suppl 1):S52, abstract 1199 J Bone Miner Res 2004; Vol 19(suppl 1):S103, abstract F497 J Bone Miner Res 2004; Vol 19(suppl 1):S103, abstract F495 |
8. Results of Trials
Serum Calcium
Study 120 (mild-moderate PHPT)
The primary endpoint of Trial 120 was the proportion of patients during maintenance
phase with normalised mean serum calcium and a mean decrease of maintenance phase
calcium of 0.125 mmol/L (0.5 mg/dL). This endpoint was met by 35/40 (88 %) patients
in the cinacalcet arm compared with 2/38 (5 %) in the placebo arm (difference = 82
%, 95 % CI 70 %, 95 %, p < 0.001, logistic regression; analysis based on LOCF.)
Study 204 (severe PHPT and PT carcinoma)
This single arm study consisted of two populations - 17 patients with intractable
PHPT and 29 patients with PT Ca. Each had a different baseline calcium level - 3.18
mmol/L and 3.53 mmol/L for intractable PHPT and PT Ca patients, respectively.
The mean serum calcium levels of patients remaining in Study 204 are shown in the
figure below.
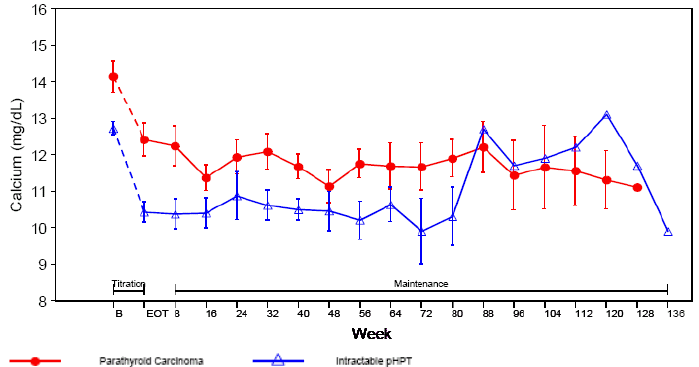
PT Ca 29 29 16 14 12 11 10 8 7 5 5 5 4 4 2 2 2 1
PHPT 17 17 14 13 9 8 8 6 5 4 3 3 1 1 1 1 1 1 1
Note: Missing data not imputed
The PBAC noted that in Study 204 (severe PHPT and parathyroid carcinoma patients)
the study population was small (17 with intractable PHPT and 29 with parathyroid carcinoma)
and that at 16 weeks a decrease in serum calcium by ? 0.25 mmol/L was achieved by
88 % of PHPT patients and 62 % parathyroid carcinoma patients. However, the mean calcium
levels by week in study 204 suggested escape in hypocalcaemic effect of cinacalcet
beyond week 80 in HPTH population, though a fall in calcium levels was then seen by
128 and 136 weeks. The reasons for this increase in calcium level were not clear from
the submission.
Other outcomes
There were statistically significant differences in the mean change (and in the mean
percent change) in PTH values between cinacalcet and placebo in Trial 120 (p < 0.019
and p < 0.009, ANOVA).
There were statistically significant increases in serum phosphorus in cinacalcet treated
patients. Other bone markers (bone alkaline phosphatase, serum N telo-peptide, and
urine N-telopeptide/creatinine ratio) also showed statistically significant changes
compared to placebo.
There was a statistically significant difference in bone mineral density (BMD) at
the lumbar spine in favour of cinacalcet at 24 weeks but not at 48 weeks. There were
no statistically significant differences between cinacalcet and placebo treated patients
in % change in BMD at the femur or forearm at 24 and 48 weeks.
The PBAC noted that the trial and studies included in the submission only presented
results for biochemistry measures and BMD. There were no data on clinical outcomes
(fractures, myocardial infarctions [MI], renal tract calculi or deaths).
There were no differences in measures of quality of life between cinacalcet and placebo
treated patients. In Trial 120, by AUC analysis no SF-36 scale or subscale showed
a statistically significant difference between cinacalcet and placebo. In Study 204,
the submission concluded that it could not be determined whether cinacalcet improved
quality-of-life overall because the patients who withdrew or were non-compliant may
have had worsened quality-of-life, had it been measured.
Nausea, myalgia, paraesthesia, dyspepsia, and hypoesthesia were more common in cinacalcet
treated patients. The Product Information notes post marketing reports of hypotension
and worsening heart failure in patients with impaired cardiac function which also
could be caused by hypocalcaemia.
9. Clinical Claim
The submission described cinacalcet as superior to placebo in
comparative effectiveness in terms of reducing serum calcium. The
submission also described cinacalcet as safe and
well-tolerated.
For PBAC's views see the Recommendations and
Reasons.
10. Economic Analysis
A stepped economic evaluation was presented for three cohorts,
mild-moderate PHPT, severe PHPT, and PT Ca. The models employed a
Markov structure with five health states. The model had a time
horizon of 34.5 years.
The results of the sensitivity analyses using the corrected model
demonstrated that the model was most sensitive to serum calcium
reduction and its relationship to mortality. The model was also
sensitive to assumptions regarding the duration of the model and
the duration of the treatment effect.
The PBAC noted the incremental base case cost-effectiveness ratios
for the severe PHPT and the parathyroid cancer population were
high, in the range $45,000 – 75,000 per QALY and $105,000
– 200,000 per QALY, respectively.
There was considered to be uncertainty associated with these
estimates including the assumption that benefits in reduction in
serum calcium levels observed over a maximum observation of four
years are assumed to continue for the 35 year duration of the model
and the extrapolation of the estimate of survival benefit, as
identified in advice to the PBAC.
11. Estimated PBS Usage and Financial Implications
The financial cost/year to the PBS was estimated to be < $10
million in Year 5.
12. Recommendation and Reasons
The PBAC noted the Pre-PBAC response had revised the requested
listing for use in primary hyperparathyroidism (PHPT) to restrict
usage to moderate to severe cases defined as two consecutive
readings of serum calcium of > 2.85 mmol/L, and therefore the
Committee considered Study 204 most relevant in support of listing
for this patient group. The Pre-PBAC Response also proposed that a
response to treatment be defined as a reduction in serum > 0.25
mmol/L rather than > 0.125 mmol/L, as proposed in the submission
to address concerns raised by the ESC about appreciable measurement
error.
The PBAC noted that in Study 204 (severe PHPT and parathyroid
carcinoma patients) the study population was small (17 with
intractable PHPT and 29 with parathyroid carcinoma) and that at 16
weeks a decrease in serum calcium by ≥ 0.25 mmol/L was achieved
by 88 % of PHPT patients and 62 % parathyroid carcinoma patients.
However, the mean calcium levels by week in study 204 suggested
escape in hypocalcaemic effect of cinacalcet beyond week 80 in HPTH
population, though a fall in calcium levels was then seen by 128
and 136 weeks. The reasons for this increase in calcium level were
not clear from the submission.
The submission presented no data on clinical outcomes such as
fracture, myocardial infarction or death, nor were there
differences in measures of QOL between active and placebo-treated
patients. The PBAC considered that due to the apparent lack of
durability of the hypocalcaemic effect, the lack of data in other
relevant outcomes and that there were no differences in quality of
life compared to placebo, the claim that cinacalcet is superior to
placebo was not reasonable.
The PBAC noted the incremental base case cost-effective ratios for
the severe PHPT and the parathyroid cancer population were high, in
the range $45,000 – 75,000 per QALY
and $105,000 – 200,000 per QALY, respectively. If the
average increase of mortality risk is used the ICERs become $>
200,000/QALY.
There was considered to be uncertainty associated with these
estimates including the assumption that benefits in reduction in
serum calcium levels observed over a maximum observation of four
years are assumed to continue for the 35 year duration of the model
and the extrapolation of the estimate of survival benefit, as
identified in advice to the PBAC. The results of the sensitivity
analyses showed the economic model is most sensitive to serum
calcium reduction and its relationship to mortality.
The PBAC therefore rejected the submission for listing for the
treatment of severe hyperparathyroidism and persistent or recurrent
hypercalcaemia following resection of parathyroid carcinoma due to
both clinical and economic uncertainties resulting in high and
uncertain cost-effectiveness ratios.
The Committee further considered whether the Rule of Rescue applied
to the requested listing for parathyroid carcinoma. The PBAC
considered that the rule did not apply as it considered there was a
lack of evidence to support the claim cinacalcet provides a
worthwhile clinical improvement sufficient to qualify as a rescue
from the medical condition.
Recommendation
Reject
13. Context for Decision
The PBAC helps decide whether and, if so, how medicines should be
subsidised in Australia. It considers submissions in this context.
A PBAC decision not to recommend listing or not to recommend
changing a listing does not represent a final PBAC view about the
merits of the medicine. A company can resubmit to the PBAC or seek
independent review of the PBAC decision.
14. Sponsor’s Comment
Amgen looks forward to working collaboratively with the PBAC and clinical experts to find a way to make the product available to patients in Australia.



