Romiplostim, powder for injection, 165 micrograms, 375 micrograms and 625 micrograms, Nplate® - March 2010
Public Summary Document for Romiplostim, powder for injection, 165 micrograms, 375 micrograms and 625 micrograms, Nplate® - March 2010
Page last updated: 02 July 2010
Public Summary Documents
Product: ROMIPLOSTIM, powder for injection, 165
micrograms, 375 micrograms and 625 micrograms,
Nplate®
Sponsor: Amgen Australia Pty Ltd
Date of PBAC Consideration: March 2010
1. Purpose of Application:
To submission sought a Section 100 (Highly Specialised Drugs
Program) listing for initial and continuing treatment of
thrombocytopenia in adult patients with chronic immune (idiopathic)
thrombocytopenic purpura (ITP) who meet certain criteria.
Highly Specialised Drugs are medicines for the treatment of chronic
conditions, which, because of their clinical use or other special
features, are restricted to supply to public and private hospitals
having access to appropriate specialist facilities.
2. Background:
At the July 2009 meeting, the PBAC rejected a submission to list
romiplostim for the initial and continuing treatment of adult
patients with chronic immune (idiopathic) thrombocytopenic purpura
who meet certain criteria because of the uncertain place in
treatment for romiplostim, uncertain clinical benefit and uncertain
and unacceptable cost-effectiveness.
3. Registration Status:
Romiplostim 375 micrograms and 625 micrograms were TGA registered
on 8 August 2008 for the indication:
For the treatment of thrombocytopaenia in adult patients with chronic immune (idiopathic) thrombocytopaenic purpura (ITP)
- who are non-splenectomised and have had an inadequate response, or are intolerant, to both corticosteroids and immunoglobulins;
- who are splenectomised and have had an inadequate response to splenectomy.
At the time of consideration, the 165 microgram vial of romiplostim
had not been approved by the TGA.
4. Listing Requested and PBAC’s View:
Section 100 (Highly Specialised Drugs)
Private hospital authority required
Initiation:
For the treatment of thrombocytopenia in adult patients with chronic immune (idiopathic)
thrombocytopenic purpura (ITP) who fulfil one of the following criteria:
a) Splenectomised patients who:
i. Have had an inadequate response to splenectomy OR
ii. Are requiring additional chronic intervention to maintain response post splenectomy where the intervention is associated with unacceptable toxicity OR
b) Non splenectomised patients who have had an inadequate response or are intolerant
to both corticosteroid therapy and immunoglobulin therapy and in whom splenectomy
is contraindicated for medical reasons.
Inadequate response is defined as a persistent platelet count of:
- ≤ 20 x 109/L or
- 20 – 30 x 109/L where the patient is experiencing bleeding or has a history of bleeding in this platelet range.
Continuation:
Patients should continue treatment if they display a sustained platelet response and
have not required more than one occasion of IVIg use during the first episode of treatment.
A sustained platelet response is defined as:
- A weekly platelet count ≥ 50 x 109/L on at least four (4) occasions; or
- A platelet count > 30 x 109/L AND a doubling of baseline platelet count, on at least four (4) occasions.
Assessment of response following the first episode of treatment would occur 24-28
weeks after initiation of therapy.
NOTE:
The following information relates to prescriptions based on the criteria described
in Initiation criteria a) ii) above. Where steroids are used to maintain a response
post splenectomy, patients may be eligible for PBS-subsidised treatment where doses
are ≥ 10 mg/day as this dose is associated with long term toxicity.
The PBAC agreed that, subject to minor amendment, the requested restriction was clinically
applicable and assisted in targeting to the most severe and needy patients who had
failed or could not undergo splenectomy. The sponsor’s proposal in the Pre-PBAC Response
concerning the dose and duration of corticosteroid therapy was considered to be reasonable,
although the PBAC did also note the variability in current practice reflected in clinician
feedback. The PBAC also considered it reasonable for the restriction to cater for
patients who have a break in therapy.
5. Clinical place for the Proposed Therapy:
Chronic immune (idiopathic) thrombocytopenic purpura (ITP) is a
long-term autoimmune disorder characterised by persistently low
platelet counts (thrombocytopenia) and cutaneous and mucosal
bleeding. Bleeding can range from mild (bruising and purpura) to
severe (intracranial or gastrointestinal haemorrhage) and can
sometimes result in death. The major therapeutic goal for ITP is to
increase platelet count to a safe level while minimising
treatment-related toxicity.
Romiplostim is a peptibody that stimulates platelet production for
long-term treatment of adult chronic ITP patients.
6. Comparator:
The submission nominated placebo as the comparator. The PBAC
considered this was appropriate for non-splenectomised patients
given the new requested restriction.
7. Clinical Trials
The re-submission re-presented the same two key direct randomised
trials as the original submission, comparing romiplostim with
placebo (both given in addition to standard care), in patients with
chronic immune thrombocytopenia purpura (ITP). One trial assessed
non-splenectomised patients, while the other trial was conducted in
splenectomised patients. Both of the key direct randomised trials
are reported in Kuter et al 2008. The re-submission also presented
new supplementary trial data in non-splenectomised patients (as
reported in Rummel et al 2009). Additional safety data from the
long term extension study of romiplostim were also presented.
The two trials presented in the submission had been published at
the time of submission, as follows:
| Trial ID/First author | Protocol title/ Publication title | Publication citation |
| Direct randomised trials | ||
| Kuter D et al (2008) | Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double-blind randomised controlled trial. | Kuter D et al, The Lancet 2008;371 (9610): 395-403 |
| Supplementary randomised trial | ||
| Rummel M et al (2009) | Efficacy and safety of romiplostim versus medical standard of care as chronic therapy for nonsplenectomized patients with immune thrombocytopenia (ITP). | Rummel M et al, Abstract for European Haematology Association 2009 |
There was uncertainty, acknowledged by the sponsor, about the
applicability of the supplementary randomised trial data to the
population for whom PBS listing was sought as the present requested
listing restricts to those patients who are either already
splenectomised or who have a significant medical contraindication
to splenectomy.
8. Results of Trials
The re-submission presented the results of post hoc analyses for the high-risk subgroup
of patients from each trial who are more representative of those for whom PBS listing
is sought; namely, those who have received at least two prior ITP therapies and who
had a baseline platelet count of less than 20×109/L, or a platelet count of 20-30×109/L with current or prior bleeding.
The objective of treatment was to prevent symptomatic bleeding by increasing platelet
count and/or improving function. It was accepted that romiplostim increased platelet
count, although there was wide variability in the response (see the interquartile
ranges below). However, the data about the effect of romiplostim on symptomatic bleeding
was reported in the trials but not used as the basis of the submission. The submission’s
cost-effectiveness analysis was based entirely on a transformation of a surrogate
(platelet response) into a clinically relevant endpoint (bleeding events).
The median platelet count at every weekly study visit for splenectomised (A) and non-splenectomised
(B) patients is shown in the following figure, taken from Kuter et al 2008:
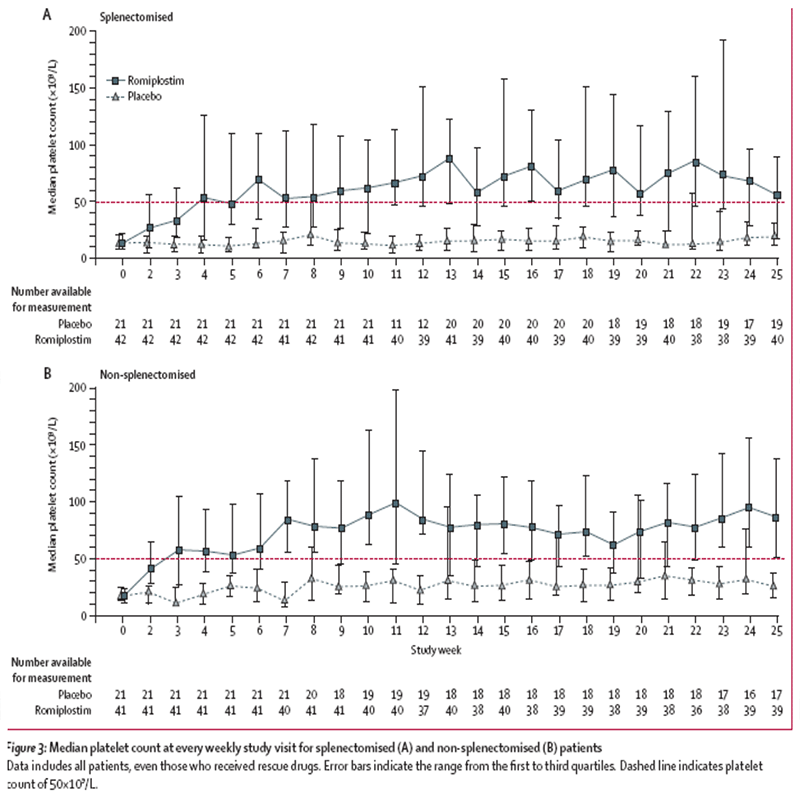
Kuter et al 2008 reported that patients given romiplostim achieved platelet counts
of 50x10(9)/L or more on a mean of 13.8 (SE 0.9) weeks (mean 12.3 [1.2] weeks in the splenectomised
group versus 15.2 [1.2] weeks in non-splenectomised group) compared with 0.8 (0.4)
weeks for those given placebo (0.2 [0.1] weeks versus 1.3 [0.8] weeks).
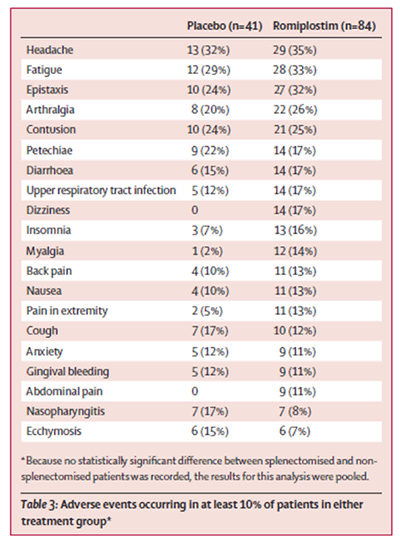
The adverse events occurring in 10 % of the patients in Kuter et al 2008 reproduced
in the figure below did not show a clear signal that romiplostim reduces the bleeding
event rates:
The submission in support of the effectiveness of romiplostim in reducing bleeding
events, presented a post hoc analysis of bleeding events from the pooled safety data
from the two key direct randomised trials and trial 131 (Rummel M, EHA 2009, abstract
1059). As the two key direct randomised trials recruited non-splenectomised patients
who did not necessarily have a contraindication to splenectomy, the majority of subjects
in this post hoc pooled analysis were not representative of the PBS population for
whom listing was requested in the re-submission.
The two key trials presented used platelet response, a surrogate outcome, rather than
a clinically relevant outcome, such as symptomatic bleeding or mortality.
The PBAC noted as previously that there was a degree of uncertainty about quantification
of the effectiveness of romiplostim in reducing clinically important bleeding, despite
evidence that romiplostim treatment results in a significant increase in the platelet
count. The tabulation of adverse events occurring in at least 10 % of patients in
either treatment group (Kuter et al, 2008) did not reveal any difference in bleeding.
However, the PBAC considered that because the reporting of these events were based
on proportions of patients ever experiencing bleeding during the trial, rather than
number of bleeding events, any benefit of romiplostim in prevention of bleeding could
not be detected. There was also uncertainty in interpreting the difference in the
trials with respect to severity of bleeding using the post hoc analysis of bleeding
related events from the pooled safety data from the two key direct randomised trials
and trial 131 (Rummel M, EHA 2009, abstract 1059). Most patients were non-splenectomised
and did not necessarily have a contraindication to splenectomy (as noted above). In
addition, the high rate of use of intravenous immunoglobulin (IVIg) use and other
rescue medication was considered likely to have reduced the number of bleeding events
in the placebo arm of the trials. Furthermore, it is biologically plausible that an
increase in platelets will result in a lowered risk of bleeding. The PBAC thus acknowledged
that the totality of the evidence suggests it is reasonable to conclude that romiplostim
reduces severe bleeding events, in patients at high risk for bleeding.
The re-submission presented updated non-comparative toxicity data from the long-term
romiplostim extension study. Adverse events (AEs), reported in 184/215 (86 %) patients,
were generally mild-moderate in severity; most common were headache (34 %), contusion
(32 %), and fatigue (31 %). Serious adverse events were reported in 29 % (62/215)
of patients. Serious AEs reported in three or more patients each were thrombocytopenia
(10/215, 7 %), increased bone marrow reticulin (5/215, 3.5 %), and congestive cardiac
failure (3/215, 2.1 %). Deaths occurred in 4 (2 %) patients; none were treatment related.
The PBAC considered that the long-term safety of romiplostim had not been adequately
established, especially in regard to the incidence and potential consequences of bone
marrow reticulin formation.
9. Claim
The re-submission claimed romiplostim as superior in terms of
comparative effectiveness to placebo, but associated with a higher
incidence of mild to moderate drug-related adverse events.
10. Economic Analysis
An updated modelled economic evaluation was presented. The economic
evaluation was altered to reflect the changes to the requested
restriction in terms of the proposed PBS population. In addition,
the results of the post hoc analysis of bleeding-related episodes
and IVIg use events were incorporated into the model.
The model reanalysed the post-hoc bleeding-related episodes (BREs)
and the subsequent effect of this on the probability of bleeding
and hospitalisation, and the rate of IVIg use. This re-analysis of
BREs increased the incremental effectiveness of romiplostim versus
placebo. As these results were derived from the post hoc
re-analysis of the non-validated post hoc outcome of BREs, their
validity was uncertain.
Restricting the proposed PBS population to high-risk chronic ITP
patients greatly reduced the incremental cost-effectiveness ratio
(ICER) for non-splenectomised patients. However, the effect on the
ICER for the post-splenectomy patients was not as pronounced
Additional sensitivity analyses related to the effect on the
incremental cost-effectiveness ratio of the rate of IVIg use were
undertaken.
The PBAC was concerned that the use of IVIg in the model, while
consistent with the trial may not be consistent with Australian
clinical practice, hence the cost-effectiveness of romiplostim in
Australia may be less favourable than calculated by the submission
on the basis of the main trial.
The method of calculating the probabilities of IVIg use was
unchanged from the initial March 2009 submission - the
probabilities of IVIg being used to treat outpatient-managed
bleeding events were calculated for the model based on IVIg usage
rates from the randomised clinical trials (RCTs). This was done by
a calibration exercise – the model inputs, probabilities of
IVIg use, were manually adjusted until the model, when run for 6
months or a year, gave IVIg usage outputs that matched usage rates
from the RCTs.
The model used 5.4 IVIg treatments per patient per year. However,
the sponsor’s own Australian data, showed that IVIg use in
those who have failed splenectomy is usually restricted to one use,
with only 36 % of patients receiving more than two such treatments
and none more than five treatments.
Sensitivity Analyses
As the sponsor did not supply the details of the calibration, it
was not possible to calculate the probability for specific rates of
IVIg use in the placebo arm. Sensitivity analyses were preformed by
assuming that the probability of IVIg use in the placebo arm is 1,
2 or 3 times the probability for the romiplostim arm (first 6
months).
With the data available, although it was possible to estimate how a
lower rate of IVIg use in the placebo arm would affect the
incremental cost, it was not possible to estimate the effect of
lowering IVIg use (and potential increased risk of bleeding events)
on health outcomes and thus incremental quality adjusted life years
(QALYs).
The submission and the presenter at the PBAC hearing for this
application argued that it was reasonable to base the rate of IVIg
use in the model on that used in the trials. The PBAC noted
concerns that data from a practice survey provided by the sponsor
suggested that the 5.4 IVIg treatments per annum for the placebo
arm in the model was not realistic in the Australian setting.
However, PBAC also noted its solicited correspondence from the
Australian Red Cross Blood Service which confirmed the existence
and size of a group of patients with severe ITP who were receiving
at least 4 IVIg treatments per annum, and averaging at least 5.4
treatments per annum. In view of this information, in conjunction
with the information presented, the PBAC considered that
romiplostim represents a cost-effective treatment for the proposed
groups of patients at an incremental cost effectiveness ratio
(ICER) in the range of $45,000 to $75,000 per QALY for
post-splenectomy patients and possibly dominant for non-splenectomy
patients.
11. Estimated PBS Usage and Financial Implications:
The submission estimated a financial cost per year to the PBS in
the range of $10 to 30 million in Year 5. This was compared with a
range of $30 to 60 million in the original submission. This
decrease was mainly due to the decrease in the number of eligible
chronic ITP patients as a result of the narrower restriction in the
requested PBS listing for the non-splenectomised subgroup.
The PBAC noted that there was considerable uncertainty about the
predicted utilisation estimates for romiplostim and there was also
concern about inappropriate use, particularly in patients who have
not undergone splenectomy.
12. Recommendation and Reasons:
The PBAC recommended listing as a pharmaceutical benefit under
section 100 (highly specialised drug) Public and Private Hospital
Authority Required for treatment of adult patients with chronic
immune (idiopathic) thrombocytopenia purpura (ITP) who meet certain
criteria, on the basis of a high but acceptable cost effectiveness
ratio, in the context of a high clinical need in a small subgroup
of ITP patients. In a previous submission, PBAC had determined that
cost-effectiveness comparison with placebo was only appropriate in
ITP patients who had failed splenectomy or in whom splenectomy was
medically contraindicated, as the sponsor had not considered
splenectomy as a comparator, nor provided data to justify the use
of romiplostim in preference to splenectomy. Although less
effective, and therefore less cost-effective, in splenectomised
patients, the PBAC noted that it was this patient group who have
the highest unmet clinical need.
The PBAC agreed that, subject to minor amendment, the requested
restriction was clinically applicable and assisted in targeting to
the most severe and needy patients who had failed or could not
undergo splenectomy. The proposal by the sponsor concerning the
dose and duration of corticosteroid therapy was considered to be
reasonable, although the PBAC did also note the variability in
current practice reflected in clinician feedback. The PBAC also
considered it reasonable for the restriction to cater for patients
who have a break in therapy.
The PBAC noted that the re-submission presented new supplementary
trial data in non-splenectomised patients. There was uncertainty,
acknowledged by the sponsor, about the applicability of the
supplementary trial data to the population for whom PBS listing is
sought as the present requested listing restricts to those patients
who are either already splenectomised or who have a significant
medical contraindication to splenectomy.
The PBAC noted as previously that there was a degree of uncertainty
about quantitation of the effectiveness of romiplostim in reducing
clinically important bleeding, despite evidence that romiplostim
treatment results in a significant increase in the platelet count.
The tabulation of adverse events occurring in at least 10 % of
patients in either treatment group (Kuter et al, 2008) did not
reveal any difference in bleeding. However, the PBAC considered
that this may have been a misrepresentation, as these events were
based on proportions of patients ever experiencing bleeding during
the trial, rather than number of bleeding events, and therefore any
benefit of romiplostim in prevention of bleeding could not be
detected. There was also uncertainty in interpreting the difference
in the trials with respect to severity of bleeding using the post
hoc analysis of bleeding related events from the pooled safety data
from the two key direct randomised trials and new supplementary
trial data (Rummel M, EHA 2009, abstract 1059). Most patients were
non-splenectomised and did not necessarily have a contraindication
to splenectomy (as noted above). In addition, the high rate of use
of intravenous immunoglobulin (IVIg) use and other rescue
medication was considered likely to have reduced the number of
bleeding events in the placebo arm of the trials. Furthermore, it
is biologically plausible that an increase in platelets will result
in a lowered risk of bleeding. The PBAC thus acknowledged that the
totality of the evidence suggests it is reasonable to conclude that
romiplostim reduces severe bleeding events, in patients at high
risk for bleeding.
The remaining uncertainty related to the rate of IVIg use, as
reduction in IVIg use represented the major cost offset in the
model, without which cost-effectiveness could not be demonstrated.
The submission and the presenter at the PBAC hearing for this
application argued that it was reasonable to base the rate in the
model on that used in the trials. PBAC noted concerns that data
from a practice survey provided by the sponsor suggested that the
5.4 IVIg treatments per annum for the placebo arm in the model was
not realistic in the Australian setting. However, PBAC also noted
its solicited correspondence from the Australian Red Cross Blood
Service which confirmed the existence and size of a group of
patients with severe ITP who were receiving at least 4 IVIg
treatments per annum, and averaging at least 5.4 treatments per
annum. In view of this information, in conjunction with information
presented, the PBAC considered that romiplostim represents a
cost-effective treatment for the proposed groups of patients at an
incremental cost effectiveness ratio (ICER) in the range of $45,000
to $75,000 per QALY for post-splenectomy patients and possibly
dominant for non-splenectomy patients.
The PBAC noted that there was considerable uncertainty about the
predicted utilisation estimates for romiplostim and there was also
concern about inappropriate use, particularly in patients who have
not undergone splenectomy. The PBAC considered that evidence that
use in a larger group of patients with less severe ITP is both
clinically necessary and cost effective should be presented before
romiplostim is subsidised for this group.
Recommendation:
ROMIPLOSTIM, powder for injection, 165 micrograms, 375 micrograms
and 625 micrograms
Restriction: Section 100 listing (Highly
Specialised Drug)
Public and Private hospital authority required
Restriction to be finalised
Pack size: 1
13. Context for Decision
The PBAC helps decide whether and, if so, how medicines should be
subsidised in Australia. It considers submissions in this context.
A PBAC decision not to recommend listing or not to recommend
changing a listing does not represent a final PBAC view about the
merits of the medicine. A company can resubmit to the PBAC or seek
independent review of the PBAC decision.
14. Sponsor’s Comment
The sponsor had no comment.



