Erlotinib, tablet, 25 mg, 100 mg, 150 mg (as hydrochloride), Tarceva® - July 2012
PDF printable version of this page (PDF 91 KB)
Public Summary Document
Product: Erlotinib, tablet, 25 mg, 100 mg, 150 mg (as hydrochloride), Tarceva®
Sponsor: Roche Products Pty Limited
Date of PBAC Consideration: July 2012
1. Purpose of Application
To extend the current Authority Required listing to include:
Initial and continuing first-line treatment, as monotherapy, of locally advanced (stage IIIB) or metastatic (stage IV) non-small cell lung cancer (NSCLC) in patients with evidence of activating mutation(s) of the epidermal growth factor receptor (EGFR) gene in tumour material who do not have progressive disease.
Or alternatively:
Initial and continuing first-line treatment, as monotherapy, of locally advanced (stage IIIB) or metastatic (stage IV) non squamous NSCLC or not otherwise specified NSCLC in patients with evidence of activating mutation(s) of the EGFR gene in tumour material who do not have progressive disease.
2. Background
Erlotinib for the first-line treatment of patients with advanced (stage IIIB) or metastatic (stage IV) non-small cell lung cancer (NSCLC) with activating epidermal growth factor receptor (EGFR) mutations and the EGFR mutation test were considered by the PBAC and the Medical Services Advisory Committee (MSAC) respectively under the pilot co-dependent technology assessment process.
After rejections in March 2006, November 2006 and a deferral in November 2007, at the March 2008 meeting, the PBAC recommended the Authority Required listing of erlotinib on the PBS for treatment of a patient with non-small cell lung cancer who meets certain criteria on the basis of acceptable cost-effectiveness compared with best supportive care at the new price proposed. Erlotinib was PBS listed on 1 August 2008 for this indication.
3. Registration Status
Erlotinib was TGA registered on 10 July 2012 for the following indication:
- the first-line treatment of patients with advanced (Stage IIIB) or
metastatic (Stage IV) non-small cell lung cancer (NSCLC) with activating EGFR mutations.
Erlotinib is also TGA registered for the following indications:
- maintenance therapy in patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) who have not progressed on first-line chemotherapy. Efficacy is influenced by tumour characteristics.
- treatment of patients with locally advanced or metastatic non-small cell lung cancer after failure of prior chemotherapy.
- Erlotinib in combination with gemcitabine is indicated for the treatment of patients with locally advanced, unresectable or metastatic pancreatic cancer.
4. Listing Requested and PBAC’s View
Based on the proposed TGA indication when a patient is diagnosed with locally advanced or metastatic NSCL
Authority Required
Initial PBS-subsidised treatment, as monotherapy, for the first-line treatment of locally advanced (stage IIIB) or metastatic (stage IV) non-small cell lung cancer in patients where there is evidence that the patient has an activating mutation(s) of the epidermal growth factor receptor (EGFR) gene in tumour material.
Authority Required
Continuing PBS-subsidised treatment, as monotherapy, of a patient who has previously been issued with an authority prescription for erlotinib and who does not have progressive disease.
Or based on the alternative limited to non squamous or not otherwise specified NSCLC:
Authority Required
Initial PBS-subsidised treatment, as monotherapy, for the first-line treatment of locally advanced (stage IIIB) or metastatic (stage IV) non-squamous non-small cell lung cancer or not otherwise specified non-small cell lung cancer in patients where there is evidence that the patient has an activating mutation(s) of the epidermal growth factor receptor (EGFR) gene in tumour material.
Authority Required
Continuing PBS-subsidised treatment, as monotherapy, of a patient who has previously been issued with an authority prescription for erlotinib and who does not have progressive disease.
For PBAC’s view, see Recommendation and Reasons.
5. Clinical Place for the Proposed Therapy
Non-small cell lung cancer (NSCLC) is the most common type of lung cancer. There are three common forms of NSCLC: adenocarcinomas (found in an outer area of the lung), squamous cell carcinomas (found in the centre of the lung next to a bronchus) and large cell carcinomas (occur in any part of the lung). Smoking causes most cases of lung cancer. The risk for lung cancer can be increased by a number of factors including passive smoking, exposure to high levels of air pollution, exposure to drinking water containing high levels of arsenic and working with or near cancer-causing chemicals or materials e.g. asbestos, uranium, beryllium, coal products, products using chloride and formaldehyde and diesel exhaust.
Epidermal growth factor receptor (EGFR) is expressed on the cell surface of a substantial percentage of NSCLCs. Activating EGFR mutations are more commonly observed in patients with adenocarcinomas and no prior history of smoking, as well as in females and those of Asian descent. The submission proposed that the place in therapy of erlotinib is to replace the most commonly used platinum-based doublet chemotherapy regimen (carboplatin and gemcitabine) as first-line treatment for locally advanced or metastatic non-small cell lung cancer (NSCLC) who have activating epidermal growth factor receptor (EGFR) mutations.
For PBAC’s view, see Recommendation and Reasons.
6. Comparator
The comparator used in the economic model in the submission was no EGFR gene mutation testing and treatment with platinum-based doublet chemotherapy after presenting with locally advanced or metastatic disease.
For PBAC’s view, see Recommendation and Reasons.
7. Clinical Trials
The submission presented two direct randomised trials to assess the comparative effectiveness and safety of erlotinib versus platinum-based doublet chemotherapy:
- EURTAC, the key direct randomised trial which compared erlotinib with platinum based chemotherapy (cisplatin or carboplatin with either docetaxel or gemcitabine) in Stage IIIB or IV NSCLC patients who had not received any prior treatment for their advanced/metastatic disease and who had activating EGFR mutations. The trial population was predominantly Caucasian; and
- OPTIMAL, the supplementary direct randomised trial which compared erlotinib with carboplatin and gemcitabine in Stage IIIB or IV NSCLC patients who had not received any prior treatment for their advanced/metastatic disease and who had activating EGFR mutations. The trial population was predominantly Asian.
The published trials and associated reports presented in the submission to assess the comparative effectiveness and safety of erlotinib versus platinum based doublet chemotherapy are presented in the following table:
|
Trial ID / First author |
Protocol title / Publication title |
Publication citation |
|
Key randomised controlled trial |
||
|
EURTAC (ML20650 |
A Phase III, multicentre, open-label, randomised study of erlotinib (Tarceva®) treatment versus chemotherapy in patients with advanced non-small-cell carcinoma of the lung who presents mutations in the tyrosine kinase (TK) domain of epidermal growth factor receptor (EGFR). |
Report Number 1041609, May 2011.
|
|
Clinical Study Report Addendum ML20650:
|
A Phase III, multicentre, open-label, randomised study of erlotinib (Tarceva®) treatment versus chemotherapy in patients with advanced non-small-cell carcinoma of the lung who presents mutations in the tyrosine kinase (TK) domain of epidermal growth factor receptor |
Report Number 1043938, July 2011 |
|
Supplementary randomised controlled trial |
||
|
Zhou C et al. (2011) |
Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study.
|
Lancet Oncology 2011; Vol. (12):735-42
|
|
Zhou C et al. (2011) |
Updated efficacy and quality-of-life (QoL) analyses in OPTIMAL, a phase III, randomised, open-label study of first-line erlotinib versus gemcitabine/carboplatin in patients with EGFR-activating mutation-positive (EGFR Act Mut+) advanced non-small cell lung cancer (NSCLC |
Journal of Clinical Oncology 2011; (Supplement): Abstract 7520
|
The direct randomised trial evidence comparing erlotinib with platinum doublet chemotherapy in the first-line treatment of EGFR mutation positive patients with locally advanced or metastatic NSCLC was linked in the submission with evidence regarding the performance of available testing methodologies to determine EGFR mutation positive status.
The components of the linked evidence for EGFR mutation testing and use of erlotinib are shown in the following table:
|
Prognostic evidence§ |
The evidence included a mix of prospective and retrospective studies. The design of one study was unclear. |
k=7 n=674 |
|
Comparative test performance |
Studies that compared different testing methodologies from archival specimens or samples from randomised controlled trials. Concordance data were presented. |
k=18 n=NR |
|
Change in patient management |
Randomised controlled trials of drug vs. usual care in patients that are biomarker positive showing that biomarker determination guides treatment with the drug. |
k=2 n=327 |
|
Treatment effectiveness |
Two open-label randomised controlled trials of drug vs. usual care in patients that are test positive in both arms. |
k=2 n=327 |
k=number of studies, n=number of patients.
There are limited data in the Australian clinical practice setting from which to judge the applicability of the data presented in the submission. The patient populations, tests used to detect the EGFR mutation, and biomarker prevalence in the presented evidence were reasonably transferable across the presented linkages dealing with prognosis, comparative analytical performance, change in management and treatment effectiveness.
8. Results of Trials
The submission presented a comparison of erlotinib with platinum doublet chemotherapy and a comparison of erlotinib with gefitinib in the event that gefitinib was PBS listed or being considered by PBAC for first-line treatment.
In the EURTAC trial, 174 eligible patients were randomised (1:1) to erlotinib (n=86) and chemotherapy (n=87). The trial design was open-label. The primary outcome of progression free survival (PFS) (with progression assessed clinically and radiologically with CAT scans every 6 weeks and PET scans as necessary) is a subjective measure and the non-blinded nature of the trial is likely to lead to observer bias. An Independent Review Committee (IRC) provided data from the EURTAC trial, but only for data cut-off date 2 August 2010; not for any updated analyses. Analysis of the primary endpoint of PFS was performed using the ITT population, and the per protocol (PP) population.
Overall, in the EURTAC trial, progression free survival was greater with erlotinib than with chemotherapy in patients with advanced EGFR mutation positive NSCLC.
The Kaplan Meier curve of PFS in the erlotinib EURTAC trial (ITT population) is shown below:
Kaplan-Meier curve of PFS in the erlotinib EURTAC trial (ITT population)
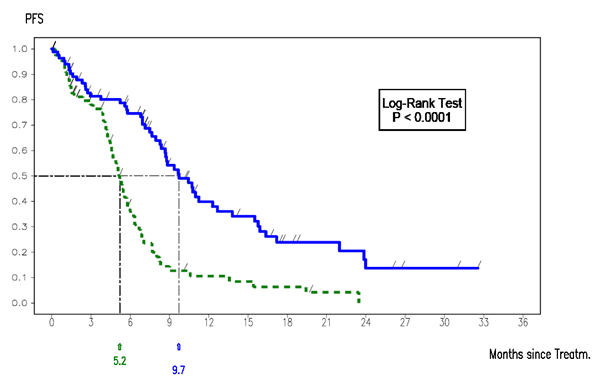
Median PFS for erlotinib = 9.7 months.
ITT=Intention-to-treat; PFS=Progression-free survival.
In the analysis by stratification factors used in the randomisation, the gain in PFS favouring erlotinib over chemotherapy was particularly large (74% reduction in risk) for patients with very good performance status (ECOG=0). The difference between the treatment arms was not statistically significant for either the ECOG =2 or the mutation Exon 21 subgroups although each trend favoured erlotinib. These subgroups had small patient numbers and likely lacked adequate statistical power.
The results of the updated analyses of PFS (95% CI) by baseline subgroups are summarised in the following figure:
Updated analysis of PFS (95% CI) by baseline subgroups (26 Jan 2011)
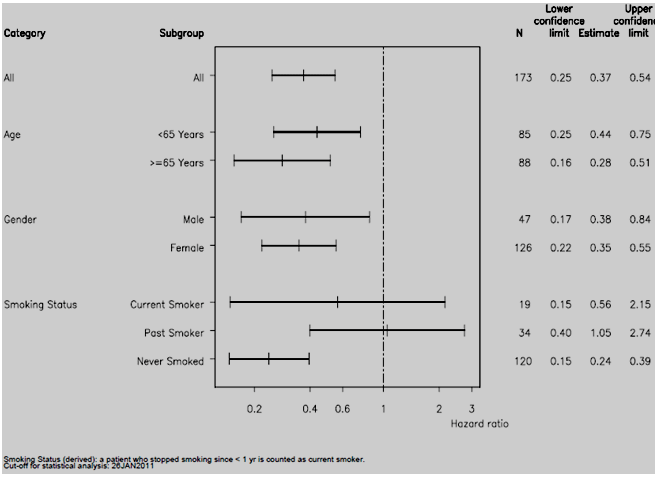
PFS was also analysed by the subgroups of histology (adenocarcinoma vs. other) and previous treatments (previous surgery, radiotherapy and chemotherapy) related to NSCLC. The HR favoured erlotinib over chemotherapy for patients with adenocarcinoma (HR=0.37, 95% CI: 0.24, 0.56), no previous surgery (HR=0.32, 95% CI: 0.21, 0.49), no previous radiotherapy (HR=0.31, 95% CI: 0.20, 0.48) and no previous chemotherapy (HR=0.35, 95% CI: 0.23, 0.52).
Overall survival was not statistically significantly different with erlotinib treatment than with chemotherapy for patients with advanced EGFR mutation positive NSCLC.
The proportion of patients who died was higher in the erlotinib arm (44%) compared to the chemotherapy arm (36%). At the time of the updated analysis for the EURTAC trial, there was a high level of cross-over to erlotinib following disease progression in the chemotherapy arm:
- A total of 67 patients (77%) in the chemotherapy arm had already received therapy compared with 38 patients (44%) in the erlotinib group. 65/67 (97%) patients in the chemotherapy arm received erlotinib subsequently.
- In the erlotinib arm, anti-metabolites (mainly pemetrexed) were administered to 30/38 patients (79%) who received subsequent treatment compared to 13/67 (19%) patients in the chemotherapy arm; and
- 27/38 (71%) of patients in the erlotinib arm received platinum-based compounds compared to 5/67 (7%) of patients in the chemotherapy arm
Because the EURTAC trial allowed for switching (cross-over) on disease progression, the results for overall survival inform a comparison of early versus late erlotinib; and they currently show no benefit (as at 26 January 2011 cut-off, log rank p = 0.8702).
For PBAC’s view of these results, see Recommendation and Reasons.
The PBAC considered that the OPTIMAL trial was less relevant to the Australian setting compared to the EURTAC trial because:
- it involved an Asian population and therefore may not represent the broader Australian population. Further, Asian origin has been shown to be a predictor of activating EGFR mutations. The ESCs noted that the EURTAC trial consisted mainly of Caucasian patients.
- the results may have potential observer bias as the trial was open label with a subjective endpoint of progression and no independent assessment of progression which makes it difficult to assess potential observer bias; and
- There is limited OS data from the trial.
In the EURTAC trial, although there was a higher proportion of deaths in the treatment phase in the erlotinib arm compared with the chemotherapy arm the submission correctly noted that this may be due to the longer treatment phase associated with erlotinib.
Adverse events were lower in the erlotinib arm compared with the chemotherapy arm for asthenia, nausea, anaemia, neutropenia, leukopenia, thrombocytopenia and tinnitus. The reverse was observed for cough and dyspnoea, rash, pruritus, skin fissures and erythematous, musculoskeletal and connective tissue disorders , infections and infestations, eye disorders, hepatobiliary disorders and cardiac disorders. Overall the safety profile of erlotinib was consistent with the known adverse effects noted in the Product Information. The safety profile of erlotinib is different from that observed with chemotherapy.
9. Clinical Claim
The submission described erlotinib as superior in terms of comparative effectiveness and “favourable” in terms of comparative safety over platinum-based doublet chemotherapy in the first-line treatment of locally advanced or metastatic NSCLC in patients with activating EGFR mutations.
For PBAC’s view, see Recommendation and Reasons.
10. Economic Analysis
The submission presented a modelled economic evaluation (a cost-utility analysis in terms of cost per quality-adjusted life-year (QALY) gained) based on a superiority claim of the proposed scenario (both EGFR testing and first-line erlotinib are available) over the current scenario (neither the EGFR testing nor first-line erlotinib is available) for both comparative benefit and harms.
The model was based on the submission base-case scenario with an ICER of between $15,000- $45,000 per QALY based on the observed progression-free survival benefit of the first-line erlotinib over doublet chemotherapy from the EURTAC trial, extrapolated to 5 years (from a median follow-up of 14 months in the trial), incorporating the cost of chemotherapies that are most commonly used in Australian clinical practice, applying utility weights from one published study, and including a maintenance therapy in the comparator arm primarily based on advice from a clinician survey.
There are three health states in the model – progression-free, progressive disease and death. All patients enter the model in the progression-free health state.
The submission claimed that the proportion of patients who are EGFR negative and the treatment that they receive will remain the same in both the proposed and comparator scenarios, hence the submission considered that the health outcomes and costs associated with EGFR negative patients can be cancelled out, and thus the economic model could be collapsed and simplified.
Kaplan-Meier estimates of both progression-free survival and overall survival are used to predict incremental costs and effectiveness. Patients commence first-line treatment in the progression-free health state. In the chemotherapy arm of the model, patients accumulate the costs of a maximum of four cycles of chemotherapy, followed by maintenance treatment with pemetrexed and erlotinib prior to disease progression. Upon disease progression, a proportion of patients receive second- and third-line treatment with pemetrexed, docetaxel, erlotinib or gefitinib. In the erlotinib arm of the model, patients continue to receive erlotinib as monotherapy (and accumulate costs) until disease progression or discontinuation due to other reasons. Upon disease progression, a proportion of patients receive treatment with either single agent or doublet chemotherapy.
The submission also claimed that the costs of second- and third-line treatments are applied as one-off costs at the time that the patient progresses in either arm of the model. In addition, the model applied a one-off incremental cost of EGFR testing to the erlotinib arm at the start of the model, as well as allowing for the cost of re-testing.
The costs associated with the most commonly used first-line chemotherapies in Australian clinical practice (carboplatin/paclitaxel and carboplatin/gemcitabine) rather than those administered in the EURTAC trial (cisplatin/gemcitabine, carboplatin/gemcitabine, carboplatin/docetaxel and cisplatin/docetaxel) are included in the current scenario arm of the model.
The summary of key sensitivity analyses showed the model is most sensitive to the prevalence of EGFR positive mutations in NSCLC, the proportion of patients receiving maintenance therapy in the comparator arm, the duration of post-progression treatment with erlotinib or gefitinib, and the proportion of patients receiving second-line treatments.
For PBAC’s view, see Recommendation and Reasons.
11. Estimated PBS Usage and Financial Implications
The likely number of patients treated per year was estimated in the submission to be less than 10,000 in Year 5 in both the submission base-case scenario and the submission alternative (i.e non squamous) scenario, at an estimated net cost per year to the Government of less than $10 million in Year 5 for both the submission base case and the submission alternative scenario.
For PBAC’s view, see Recommendation and Reasons.
12. Recommendation and Reasons
The PBAC requested advice from MSAC on the disease stage at which subsidised testing should occur, the total number of tests, the number of tests per patient reflecting the frequency of repeat testing, the costs of testing per patient treated with first-line erlotinib, and the overall increase in the cost of testing to support first-line use compared to current testing for the existing PBS listing of gefitinib effectively as third-line therapy. The prevalence of EGFR mutations in Australian patients with NSCLC for both a pre-selected “enriched” population excluding squamous cell NSCLC and an unselected population may be particularly important given the consequences for both the cost-effectiveness and the financial implications of the proposal.
The PBAC noted that a consequence of recommending the extended listing as proposed would be a need to reconsider whether the existing PBS listing of erlotinib should be similarly limited to patients with an EGFR activating mutation. In doing so, the PBAC considered that current practice reflects second-line use in patients selected on clinical grounds to have NSCLC that is more likely to be mutation positive, and that ascertaining a response to the drug is being used in place of ascertaining the mutation status of a patient’s cancer.
The PBAC compared this submission for erlotinib with the submission it rejected on the grounds of unacceptably high and uncertain cost-effectiveness in November 2010 for gefitinib to treat the same requested population. It also considered the application against the information it had requested to judge the co-dependency between EGFR mutation testing and drug performance and the factors influencing this co-dependency.
The PBAC noted that there are effective alternative therapies in the requested first-line setting. This means that ineffective use of erlotinib in patients without an activating mutation would result in a net harm for patients, as indicated by the early stopping of the TORCH trial due to a statistically significant worsening of overall survival with first-line erlotinib compared with platinum-based chemotherapy. In that trial, patients were not selected as having mutation positive NSCLC. Thus the consequences of false positive test results are particularly important in the first-line setting. In this regard, the PBAC noted the potentially important omission in the submission of a model that was capable of examining the consequences of varying test accuracy. Therefore the advice of MSAC was also sought on the extent of discordance across the various EGFR test options.
The PBAC accepted that the overall comparison, as reflected in the key EURTAC randomised trial, was first-line chemotherapy with the option of erlotinib after disease progression versus first-line erlotinib with the option of chemotherapy after disease progression. The PBAC also considered that there might be some substitution for best supportive care given erlotinib’s different symptomatic side-effect profile compared with chemotherapy. However, the PBAC rejected the submission’s inclusion of maintenance therapy in only the first-line chemotherapy arm of the model. The use of erlotinib for this purpose is specifically excluded by its current PBS restriction, and although this use of pemetrexed is less clearly excluded by its current PBS restriction, it can also be claimed to be part of current practice and therefore should also have been applied to the first-line erlotinib arm of the model. The submission’s inclusion of maintenance therapy in only the first-line chemotherapy arm of the model is important because the model approach results in apparently favourable cost-effectiveness for the proposal. However it achieves this by adding disproportionately increased costs of the comparator arm compared to increased health benefits, reflecting the PBAC view that this included maintenance therapy is not acceptably cost-effective. The PBAC noted again that it had not assessed the cost effectiveness of maintenance therapy in NSCLC and, as noted above, the relevant drug restrictions had been designed to make this clear.
The PBAC noted that the key EURTAC trial in mutation positive patients suggested a statistically significant and potentially clinically important benefit for erlotinib monotherapy over platinum-based chemotherapy in terms of an additional median progression-free survival of approximately 4.5 months (log rank p < 0.0001, 26 January 2011 cut-off) compared to platinum chemotherapy. Because the EURTAC trial allowed for switching (cross-over) on disease progression, the results for overall survival inform a comparison of early versus late erlotinib; and they currently show no benefit (as at 26 January 2011 cut-off, log rank p = 0.8702). The PBAC noted that the comparison of early versus late erlotinib is relevant in the Australian context because erlotinib is currently listed second-line in unselected patients. Thus the clinical benefit for EGFR activating mutation positive patients of listing erlotinib as first-line treatment in addition to second-line treatment is an improvement in quality of life, but not a prolongation of life.
The modelled economic evaluation which extrapolated beyond the trial horizon appropriately did not include an improvement in overall survival. The incremental cost effectiveness per extra QALY gained of erlotinib (without including maintenance therapy in Step 4 of the economic evaluation) at its existing price is an unacceptable $105,000 – $200,000.
The PBAC agreed that other aspects of the modelled economic evaluation also favoured erlotinib, including the proportion of patients taking up second-line therapy (80% would better reflect regular clinical practice compared with the 97% derived from the trials) and the duration of this second-line therapy, the prevalence of activating mutations in the tested population, and the smaller effect of different sources of utilities in the translation of prolonged progression-free survival to incremental QALY gains via utility differences in the health states of the model.
The financial implications are also uncertain, with a net increased annual cost to government ranging from the submission’s estimate of less than $10 million per year to higher, though in the same range using the alternative estimates. Of these, the higher estimates are more likely unless there is also a corresponding reduction in erlotinib use in second-line by restricting its listing to patients who have NSCLC with an EGFR activating mutation.
The PBAC therefore rejected the application to extend the listing of erlotinib at its current price to the first-line setting of locally advanced or metastatic NSCLC on the grounds of unacceptably high and uncertain cost-effectiveness, noting that erlotinib is already listed and the expected lack of any overall survival gain for patients resulting from the proposed extended listing.
The PBAC also acknowledged and noted the consumer comments on this item.
Recommendation:
Reject
13. Context for Decision
The PBAC helps decide whether and, if so, how medicines should be subsidised in Australia. It considers submissions in this context. A PBAC decision not to recommend listing or not to recommend changing a listing does not represent a final PBAC view about the merits of the medicine. A company can resubmit to the PBAC or seek independent review of the PBAC decision.
14. Sponsor’s Comment
The sponsor supports the decision of the PBAC to hold a Stakeholder Meeting with clinicians, pathologists, consumer and industry representatives to help address the outstanding matters of concern regarding who, when, what and how to test for the EGFR mutation in the first-line NSCLC setting. The sponsor intends to address the economic issues within a resubmission, including the impact of diagnostic accuracy (including additional concordance data across test methodologies) and maintenance therapy on the cost-effectiveness of erlotinib in previously untreated EGFR-positive NSCLC.



