Galsulfase-rch, solution concentrate for I.V. infusion, 5 mg in 5 mL, Naglazyme, July 2007
Public summary document for Galsulfase-rch, solution concentrate for I.V. infusion, 5 mg in 5 mL, Naglazyme, July 2007
Page last updated: 09 November 2007
PDF version of this page (PDF 42KB)
Public Summary Documents
Product: Galsulfase-rch, solution concentrate for I.V. infusion, 5 mg in 5 mL, Naglazyme
Sponsor: BioMarin Pharmaceutical Inc.
Date of PBAC Consideration: July 2007
1. Purpose of Application
The submission requested listing of galsulfase-rch as an authority required benefit for the treatment of mucopolysaccharidosis type VI (MPS VI). If the PBS listing were to be rejected on cost-effectiveness grounds, the submission sought for the drug to be considered for the Life Saving Drugs Program (LSDP).
2. Background
This drug had not previously been considered by the PBAC.
Background to the LSDP:
The Commonwealth Government provides funds under an appropriation item established
for the specific purpose of assisting access to expensive and lifesaving drugs accepted
by the PBAC as clinically effective, but not available as pharmaceutical benefits
because of a failure to meet cost effectiveness criteria. Financial assistance for
such drugs is approved in accordance with specified eligibility criteria and subject
to certain conditions as agreed by the Ministers for Health and Finance.
3. Registration Status
Galsulfase-rch was TGA approved for marketing on 16 February 2007 for the treatment of mucopolysaccharidosis VI (MPS VI).
4. Listing Requested and PBAC’s View
Authority required
For use in patients who have a positive diagnosis of mucopolysaccharidosis type VI
(MPS VI), as detailed below.
Criteria for admission to treatment
Diagnosis of MPS VI
- The diagnosis of MPS VI must have been established by the demonstration of specific deficiency of the lysosomal enzyme arylsulfatase B (ASB; also called N-cetylgalatosamine-4-sulfatase) consistent with the newly approved Australian treatment guidelines.
See Recommendation and Reasons for PBAC’s view.
5. Clinical Place for the Proposed Therapy
MPS VI is an inherited lysosomal storage disorder. It is characterised by a deficiency
of glycosaminoglycan (GAG)-degrading lysosomal enzymes. The disease severity ranges
from rapidly progressing to slowly progressing, but all with foreshortened lives.
These patients ultimately become wheelchair bound or bedridden secondary to skeletal
deformities, joint disease, cardiopulmonary disease, blindness and spinal cord compression.
Galsulfase-rch offers an enzyme replacement therapy for MPS VI.
6. Comparator
Appropriately the submission nominated placebo (plus standard medical management) as the comparator.
7. Clinical Trials
The submission presented:
- one key head-to-head randomised comparative trial comparing 1mg/kg galsulfase solution (with standard medical management, SMM) and placebo solution (with SMM) in patients with MPS VI, over 24 weeks (Harmatz et al, 2006);
- one supporting non-randomised study of 1mg/ kg galsulfase solution in patients with MPS VI (Harmatz et al, 2005)
- one supporting randomised trial of 1mg/ kg galsulfase solution versus 0.2mg/kg galsulfase solution in six patients with MPS VI (Harmatz et al, 2004); and
- three supporting oral presentations by P. Harmatz which present pooled (unweighted) results from the three Harmatz trials above.
These trials have been published as:
|
Trial/First author |
Protocol title |
Publication citation |
|---|---|---|
|
Harmatz et al (2006) |
A phase 3, randomized, double-blind, placebo-controlled, multicenter, multinational clinical study of recombinant human N-acetylgalactosamine 4-sulfatase (rhASB) in patients with mucopolysaccharidosis VI. |
The Journal of Paediatrics 2006; 148:533-9. |
|
Supporting |
||
|
Harmatz et al (2005) |
Direct comparison of measures of endurance, mobility, and joint function during enzyme-replacement therapy of Mucopolysaccharidosis VI (Maroteaux-Lamy Syndrome): Results after 48 weeks in a phase 2 open-label clinical study of recombinant human N-acetylgalactosamine 4-sulfatase. |
Paediatrics 2005; 115:e681-e689. |
|
Harmatz et al (2004) |
Enzyme replacement therapy in Mucopolysaccharidosis VI (Maroteuax-Lamy Syndrome) |
The Journal of Paediatrics 2004; 144:574-80. |
|
McGill (2006) |
Enzyme replacement therapy for MPS VI with recombinant human N-acetylgalactosamine 4-sulphatase (rhASB) from 8 weeks of age – a sibling control study |
Presented at the International Congress of Inborn Errors of Metabolism September 2006, Japan. |
|
Harmatz (presentations) |
Long-term enzyme replacement therapy (ERT) with Naglazyme™ (galsulfase) for MPS VI:
Growth, pubertal development, and pulmonary function |
Presented at the International MPS Society Meeting, 1st July 2006 Venice, Italy |
8. Results of Trials
Key trial
Distance walked during the 12 minute walk test at 24 weeks, Harmatz et al (2006)
|
Galsulfase plus SMM (m) |
Placebo plus SMM |
Mean difference (SE) |
|||
|---|---|---|---|---|---|
|
n |
mean (SD) |
n |
mean (SD) |
||
|
Observed data |
|||||
|
Baseline |
19 |
227 (170)a |
20 |
381 (202)b |
-154 (60) |
|
Week 6 |
19 |
290 (201) |
19 |
383 (213) |
-93 (67) |
|
Week 12 |
19 |
303 (216) |
19 |
398 (208) |
-94 (69) |
|
Week 18 |
18c |
344 (202) |
19 |
399 (226) |
-55 (70) |
|
Week 24 |
19 |
336 (227)d |
19 |
399 (217)e |
-63 (72) |
|
Fitted data (adjusted for baseline) |
|||||
|
mean (95% CI) |
mean (95% CI) |
mean difference (95% CI) |
|||
|
Baseline |
19 |
306 |
20 |
306 |
0 |
|
Week 6 |
19 |
378 (322, 434) |
19 |
316 (263, 369) |
62 (-18, 142) |
|
Week 12 |
19 |
392 (336, 447) |
19 |
331 (278, 384) |
61 (-20, 141) |
|
Week 18 |
18c |
421 (365, 478) |
19 |
332 (279, 385) |
89 (9, 170) |
|
Week 24 |
19 |
424 (368, 480) |
19 |
332 (280, 385) |
92 (11, 172) |
amedian 210m; b median 365m; c one patient did not perform the test because of a serious adverse event; d median 316m; e median 373m; SMM = standard medical management; bold indicates the primary endpoint
Results for the distance walked in 12 minutes at 48 weeks for the key trial (Harmatz
et al, 2006) are presented in the figure below (as change from baseline). Weeks 24-48
represent the open-label period when all patients were treated with galsulfase solution
(plus SMM). Patients in the placebo (plus SMM) group could walk significantly further
than patients in the galsulfase group at baseline (p = 0.014).
Distance walked (metres) during the 12 minute walk test at 48 weeks, Harmatz et al
(2006)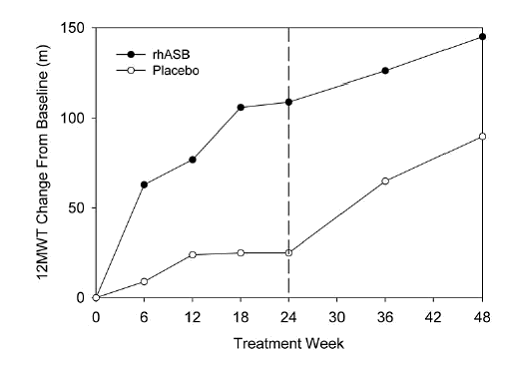
rhASB = galsulfase
The PBAC agreed that galsulfase (plus SMM) appears to have statistically significant
and clinically important advantages in effectiveness over placebo (plus SMM) for the
primary outcome, the twelve minute walk test (12MWT) over a 24 week time horizon.
The published report stated that there was a statistically significant, mean improvement
in favour of galsulfase (plus SMM) compared with placebo (plus SMM) for the 6MWT at
Week 24 (53m: 95% CI; 14, 90; and p-value 0.007).
The table below summarises the rate at which stairs were climbed during the three
minute stair climb (3MSC) in the key trial (Harmatz et al, 2006).
Rate at which stairs were climbed during the 3 minute stair climb at 24 weeks, Harmatz
et al (2006)a
|
Galsulfase plus SMM (stairs/minute) |
Placebo plus SMM (stairs/minute) |
Mean difference (95% CI) |
|||
|---|---|---|---|---|---|
|
N |
mean (95% CI) |
N |
mean (95% CI) |
||
|
Baseline |
19 |
25.4 |
20 |
25.4 |
0 |
|
Week 6 |
19 |
31.5 (27.4, 35.6) |
19 |
26.7 (22.8, 30.6) |
4.8 (-1.0, 10.6) |
|
Week 12 |
19 |
33.1 (29.0, 37.2) |
18 |
27.2 (23.2, 31.1) |
5.9 (0.1, 11.7) |
|
Week 18 |
18 |
35.0 (30.9, 39.1) |
19 |
28.1 (24.2, 32.0) |
6.9 (1.1, 12.7) |
|
Week 24 |
19 |
33.7 (29.6, 37.7) |
19 |
27.9 (24.0, 31.8) |
5.7 (-0.1, 11.5)b |
a fitted data, adjusted for baseline; b p-value = 0.053; SMM = standard medical management
There was a trend (not reaching statistical significance) towards a faster rate of
climbing stairs in patients treated with galsulfase (plus SMM) compared to placebo
(plus SMM).
There was a statistically significant, mean improvement in favour of galsulfase (plus
SMM) compared with placebo (plus SMM) for the number of steps climbed in three minutes
at Week 24 (16.37: 95% CI; 0.62, 31.9; and p-value 0.042).
The changes in glycosaminoglycan (GAG) urinary excretion rates in the key trial are
presented below (secondary outcome).
Changes in urinary GAG levels in the key trial (Harmatz et al, 2006)a
|
Galsulfase plus SMM |
Placebo plus SMM |
Mean difference (SE) |
|||
|---|---|---|---|---|---|
|
N |
mean (SD) |
N |
mean (SD) |
||
|
Baseline |
19 |
346 (128) |
20 |
330 (114) |
17 (39) |
|
Week 1 |
18 |
329 (131) |
19 |
367 (134) |
-38 (43) |
|
Week 4 |
19 |
116 (48) |
20 |
348 (126) |
-232 (30) |
|
Week 6 |
19 |
103 (45) |
19 |
339 (95) |
-237 (24) |
|
Week 8 |
19 |
94 (47) |
19 |
354 (107) |
-260 (27) |
|
Week 12 |
19 |
99 (52) |
19 |
381 (218) |
-282 (51) |
|
Week 18 |
19 |
91 (44) |
19 |
349 (199) |
-258 (47) |
|
Week 24 |
19 |
85 (35) |
19 |
317 (80) |
-232 (20) |
a observed data unadjusted for baseline; GAG = glycosaminoglycan; SMM = standard medical
management
Patients treated with galsulfase (plus SMM) experienced a 75% reduction from baseline
in GAG levels at Week 24. Adjusted for baseline, the analysis of variance showed an
estimated mean difference at Week 24 between placebo (plus SMM) treated patients and
galsulfase (plus SMM) treated patients of –22718 ?g/mg creatinine (p <0.001). 89.5%
(17/19) and 0% of patients treated with galsulfase (plus SMM) and placebo (plus SMM)
respectively experienced ?50% reduction in baseline GAG levels (difference in proportions:
0.67, 0.99; p-value 0.001). Based on the evidence provided in the submission, urinary
GAG levels were not yet an established surrogate outcome for morbidity and mortality
in MPS VI disease.
The Committee also agreed that treatment with galsulfase is associated with significant
reductions in urinary glycosaminoglycan (GAG) excretion rates, but that the clinical
importance of this finding is less clear.
The PBAC noted that the submission reported several other outcomes, including respiratory,
cardiac and musculoskeletal function, all of which failed to show any significant
advantage of galsulfase over placebo. The Committee considered that the failure of
the key trial (Harmatz et al, 2006) to show significant advantages of galsulfase over
placebo in the secondary outcome measures of respiratory, cardiac and musculoskeletal
function may in part be explained by the heterogeneity of the patient group at study
entry, with some patients already having developed joint contractions and other sequelae
of MPS VI, which might limit their ability to show improvements in these parameters.
9. Clinical Claim
The submission claimed that galsulfase had significant advantages in effectiveness over placebo (plus standard medical management) and had similar or less toxicity. The PBAC partially accepted this claim. See Recommendations and Reasons.
10. Economic Analysis
A preliminary (trial-based) economic evaluation was presented. The choice of the cost-consequences
approach was considered valid. The resources included were: pre-treatment drug costs;
galsulfase solution; galsulfase administration; serious adverse events requiring hospitalisation;
and adverse events requiring surgical and diagnostic procedures. The overall comparative
costs and outcomes for each alternative and the incremental costs and outcomes are
summarised below.
On average, each patient treated with galsulfase (plus SMM) instead of placebo (plus
SMM) over 24 weeks:
- generates additional costs of < $10 million over that period;
- can walk an additional 92 metres in 12 minutes;
- can walk an additional 53 metres in 6 minutes;
- can climb an additional 16.3 steps in 3 minutes;
- can climb an additional 5.7 stairs per minute for 3 minutes;
- experiences a reduction of 227ug/mg in urinary GAG;
- experiences a reduction of 0.53 hospitalisations; and
- experiences a reduction of 0.237 surgical or diagnostic procedures
A modelled economic evaluation was not presented. The justification for not doing
so was that a modelled economic evaluation would not provide additional information
to better appreciate the cost-effectiveness of galsulfase in patients with MPS VI
given (i) the length of the key trial (24 weeks) limits assessment of patient survival
and (ii) mortality rates for various surgeries for treatment of patients with MPS
VI were not identified in the literature.
The Committee agreed that it was appropriate for the submission not to present a modelled
economic evaluation but considered that the results of the preliminary economic evaluation
indicate that treatment with galsulfase is associated with an unacceptably high incremental
cost effectiveness ratio.
11. Estimated PBS Usage and Financial Implications
The financial cost/year to the PBS was estimated to be < $10 million in Year 4. The PBAC considered this to be a likely over-estimate in the submission.
12. Recommendation and Reasons
The Committee agreed that placebo plus standard medical management (SMM) is the appropriate
comparator for galsulfase, and that, as indicated at the sponsor’s hearing, bone marrow
transplantation represents a very poor option for patients with mucopolysaccharidosis
type VI (MPS VI) and is therefore not a valid comparator because it is not the therapy
likely to be replaced most in clinical practice
The PBAC agreed that galsulfase (plus SMM) appears to have significant and clinically
important advantages in effectiveness over placebo (plus SMM) for the primary outcome,
the twelve minute walk test (12MWT) over a 24 week time horizon, but that it has more
toxicity, mainly due to the incidence of infusion related adverse events. The Committee
also agreed that treatment with galsulfase is associated with significant reductions
in urinary glycosaminoglycan (GAG) excretion rates, but that the clinical importance
of this finding is less clear. The Committee considered that the failure of the key
trial (Harmatz et al, 2006) to show significant advantages of galsulfase over placebo
in the secondary outcome measures of respiratory, cardiac and musculoskeletal function
may in part be explained by the heterogeneity of the patient group at study entry,
with some patients already having developed joint contractions and other sequelae
of MPS VI, which might limit their ability to show improvements in these parameters.
The PBAC further accepted that the longer term effectiveness and toxicity of galsulfase
and the impact of treatment on disease progression and mortality rates are unknown.
However, as discussed at the hearing, a precedent did exist in that the very few successful
bone marrow transplants in patients with MPS VI resulted in reduced urinary GAG excretion
rates and improved survival. Overall, the Committee considered it sufficiently likely
that treatment with galsulfase would be associated with improved survival in MPS VI.
The Committee agreed that it was appropriate for the submission not to present a modelled
economic evaluation but considered that the results of the preliminary economic evaluation
indicate that treatment with galsulfase is associated with an unacceptably high incremental
cost effectiveness ratio.
The PBAC therefore rejected the application to list galsulfase on the PBS on the basis
of unacceptably high cost-effectiveness. However the Committee indicated that the
use of galsulfase for the treatment of mucopolysaccharidosis type VI meets the criteria
for the Life Saving Drugs Program (LSDP), given that it produced a clinical benefit
in terms of the 12 MWT and that it was plausible that the reduction in GAG levels
will be associated with a survival benefit.
The PBAC therefore recommended that Government considers including galsulfase on the
LSDP.
Recommendation
Reject.
13. Context for Decision
The PBAC helps decide whether and, if so, how medicines should be subsidised in Australia. It considers submissions in this context. A PBAC decision not to recommend listing or not to recommend changing a listing does not represent a final PBAC view about the merits of the medicine. A company can resubmit to the PBAC or seek independent review of the PBAC decision.
14. Sponsor’s Comment
The sponsor welcomes the Pharmaceutical Benefits Advisory Committee’s (PBAC) recommendation
to include Naglazyme in the Life Saving Drugs Program (LSDP).



