Ivacaftor, 150 mg tablet, 56, Kalydeco® - March 2014
PDF printable version of this page
Public Summary Document
Product: Ivacaftor, 150 mg tablet, 56, Kalydeco®
Sponsor: Vertex Pharmaceuticals (Australia) Pty Ltd
Date of PBAC Consideration: March 2014
1. Purpose of Application
The major submission requested a Section 100 (Highly Specialised Drugs Program) listing or inclusion on the Life Saving Drugs Program (LSDP) for treatment of cystic fibrosis (CF) in patients aged six years and older who have a class III (gating) mutation in the cystic fibrosis transmembrane regulator (CFTR) gene.
Highly Specialised Drugs Program:
Highly Specialised Drugs are medicines for the treatment of chronic conditions, which, because of their clinical use or other special features, are restricted to supply to public and private hospitals having access to appropriate specialist facilities.
Life Saving Drugs Program:
Through the Life Saving Drugs Program (LSDP), the Australian Government provides subsidised access, for eligible patients, to expensive and potentially life saving drugs for very rare life-threatening conditions.
Before a drug is made available on the LSDP it must generally be accepted by the Pharmaceutical Benefits Advisory Committee as clinically necessary and effective, but not recommended for inclusion on the Pharmaceutical Benefits Scheme due to unacceptable cost-effectiveness
2. Background
Ivacaftor is currently TGA registered for the treatment of cystic fibrosis (CF) in patients age 6 years and older who have a G551D mutation in the CFTR gene.
The PBS indication for other class III (gating) mutations (i.e. G178R, G551S, S549N, G970R, G1244E, S1251N, S1255P, G1349D) was sought under the TGA/PBAC Parallel Process. At the time of PBAC consideration, a TGA Delegate’s Overview was not available.
The PBAC considered a major submission for ivacaftor in July 2013. The PBAC noted that it may be possible to reduce the dose of ivacaftor in clinical practice by co-administering ivacaftor with a strong CYP3A inhibitor, but that even with a dose reduction, the cost per QALY would remain too high. The PBAC decided to defer making a recommendation to allow the sponsor the opportunity to consider the Committee’s views and to submit a new price proposal for PBS listing.
In November 2013 the PBAC considered a minor submission in which the sponsor indicated it was not supportive of a regimen of co-administering ivacaftor with a strong CYP3A inhibitor. The PBAC noted however that CYP3A4 inhibitors such as macrolide antibiotics are an appropriate option as boosting agents where a patient already requires these inhibitors as part of their standard CF management. The PBAC considered that patients requiring prophylactic antibiotics could plausibly comprise a substantial proportion of CF patients.
In November 2013 the PBAC recommended ivacaftor for listing. The PBAC expressed the view that ivacaftor would not be cost-effective under the sponsor’s pricing proposal. The PBAC considered that the cost-effectiveness of ivacaftor would be acceptable if the incremental cost-effectiveness ratio (ICER) was between $60,000 and $80,000 per quality-adjusted life-year (QALY) gained, and if certain risk-sharing arrangements were implemented. This ICER would be comparable with that of other recent medicines that were first in class.
The PBAC also considered that it would be desirable to put in place with the sponsor arrangements for data collection, and ensure the implementation of assumptions regarding reduction in the price of ivacaftor included in the sponsor’s model.
Public summary documents for the PBAC’s previous considerations of ivacaftor are available on the PBS website.
3. Registration Status
Ivacaftor was TGA-registered on 9 July 2013 for the treatment of cystic fibrosis (CF) in patients age 6 years and older who have a G551D mutation in the CFTR gene.
4. Listing Requested and PBAC’s View
Section 100 (Highly Specialised Drugs Program)
Authority required
OR
Life Saving Drugs Program
Treatment of cystic fibrosis in patients age six years or older who have a confirmed class III (gating) mutation in the CFTR gene.
The PBAC noted that the resubmission requested listing for patients with any Class III gating mutation of the CFTR gene. The PBAC recalled that the previous submission had requested listing only for patients with a G551D mutation of the CFTR gene, and noted that the indication for other Class III gating mutations is currently being progressed under the Therapeutic Goods Administration (TGA)-PBAC Parallel Process. The PBAC noted that as a positive TGA Delegate’s Overview for other Class III gating mutations was not available at the time of consideration, the restriction should specify only patients with a G551D mutation. Once the sponsor is able to make a resubmission with the TGA documentation for other Class III gating mutations, the PBAC indicated it would consider amending the restriction to include those patients at that time.
The submission requested listing on a cost-effectiveness basis with best supportive care (BSC).
5. Clinical Place for the Proposed Therapy
Ivacaftor is intended to be used as an add-on to current best supportive therapy, and has a different mechanism of action to the antibiotics and mucolytics currently available through the PBS for treatment of patients with cystic fibrosis.
Under the proposed listing, CF patients aged 6 and over with a confirmed class III (gating) mutation in the CFTR gene would be eligible for ongoing ivacaftor therapy. The PBAC noted that this proposed listing was slightly broader than in the previous submission, which limited therapy to those with a G551D mutation.
As above, the PBAC noted that the indication for other Class III gating mutations is still being considered by the TGA. The PBAC advised that it would reconsider the indication for other Class III gating mutations once the sponsor is able to resubmit with TGA documentation for that indication.
6. Comparator
The resubmission nominated best supportive care (BSC) as the comparator. This was previously accepted by the PBAC.
7. Clinical Trials
The July 2013 submission presented two randomised trials comparing ivacaftor with best supportive care in patients with CF involving a G551D mutation in the CFTR gene. These were the STRIVE trial (n=161, patients aged 12 years and older) and the ENVISION trial (n=52, patients aged 6-11 years), both of which ran for 48 weeks. These trials are supported by an open label extension trial (PERSIST) for both ivacaftor and placebo patients from the two key trials.
The resubmission presented extended effectiveness and safety data from PERSIST.
The published trials and associated reports presented in the resubmission are shown in the following table:
|
Clinical trial ID |
Publications/clinical study reports |
|---|---|
|
Pivotal trials |
|
|
VX08-770-102 (NCT00909532) STRIVE |
Publication: Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Drevinek P, Griese M, McKone EF, Wainwright CE, Konstan MW, Moss R, Ratjen F, Sermet-Gaudelus I, Rowe SM, Dong Q, Rodriguez S, Yen K, Ordonez C, and Elborn JS. (2011) A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. New England Journal of Medicine 365:1663-1672. Clinical study report VX08-770-102: A phase 3, randomised, double-blind, placebo-controlled, parallel-group study to evaluate the efficacy and safety of VX 770 in subjects with cystic fibrosis and the G551D mutation. |
|
VX08-770-103 (NCT00909727) ENVISION |
Clinical study report VX08-770-103: A phase 3, 2-part, randomised, double-blind, placebo-controlled, parallel-group study to evaluate the pharmacokinetics, efficacy, and safety of VX-770 in subjects aged 6 to 11 years with cystic fibrosis and the G551D mutation. |
|
Supportive trials |
|
|
VX08-770-105 (NCT01117012) PERSIST |
Clinical study synopsis. Interim analysis #2 VX08-770-105: An Open-Label, Rollover Study to Evaluate the Long-Term Safety and Efficacy of VX-770 in Subjects with Cystic Fibrosis |
|
VX06-770-101 (NCT00457821) |
Publication: Accurso FJ, Rowe SM, Clancy JP, Boyle MP, Dunitz JM, Durie PR, Sagel SD, Hornick DB, Konstan MW, Donaldson SH, Moss RB, Pilewski JM, Rubenstein RC, Uluer AZ, Aitken ML, Freedman SD, Rose LM, Mayer-Hamblett N, Dong Q, Zha J, Stone AJ, Olson ER, Ordonez CL, Campbell PW, Ashlock MA, and Ramsey BW. (2010) Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. New England Journal of Medicine 363:1991-2003. |
|
VX10-770-106 (NCT01262352) Study 106 |
Clinical study report VX10-770-106: A phase 2, Randomised, Double-Blind, Placebo-Controlled, Cross-over Study to Evaluate the Effect of VX-770 on Lung Clearance Index in Subjects With Cystic Fibrosis, the G551D Mutation, and FEV1 >90% Predicted |
The PBAC recalled that at its consideration of ivacaftor in November 2013, 687 consumer comments were provided via the PBS website. The PBAC noted the advice of Cystic Fibrosis Australia that these comments were to stand for the current agenda.
The sponsor did not request a hearing.
The PBAC previously recommended the monitoring of sweat chloride as a measure of adherence, noting that the reliability of this measure as a marker for treatment response, rather than adherence, was not known. The PBAC noted the revised advice of the Thoracic Society of Australia and New Zealand (TSANZ) that sweat chloride alone may yield spurious results. The TSANZ also highlighted issues of capacity in hospitals to administer the test to all eligible patients, the need to limit the spread of multi-resistant organisms among patients at testing sites and the cost and inconvenience of repeated testing for a lifelong treatment. The PBAC agreed that sweat chloride testing in isolation may not adequately represent long-term adherence to treatment. The PBAC also noted the possibility that a patient may exhibit a clinical response in terms of forced expiratory volume in 1 second (FEV1) and other measures, yet not satisfy the proposed criteria for sweat chloride.
8. Results of Trials
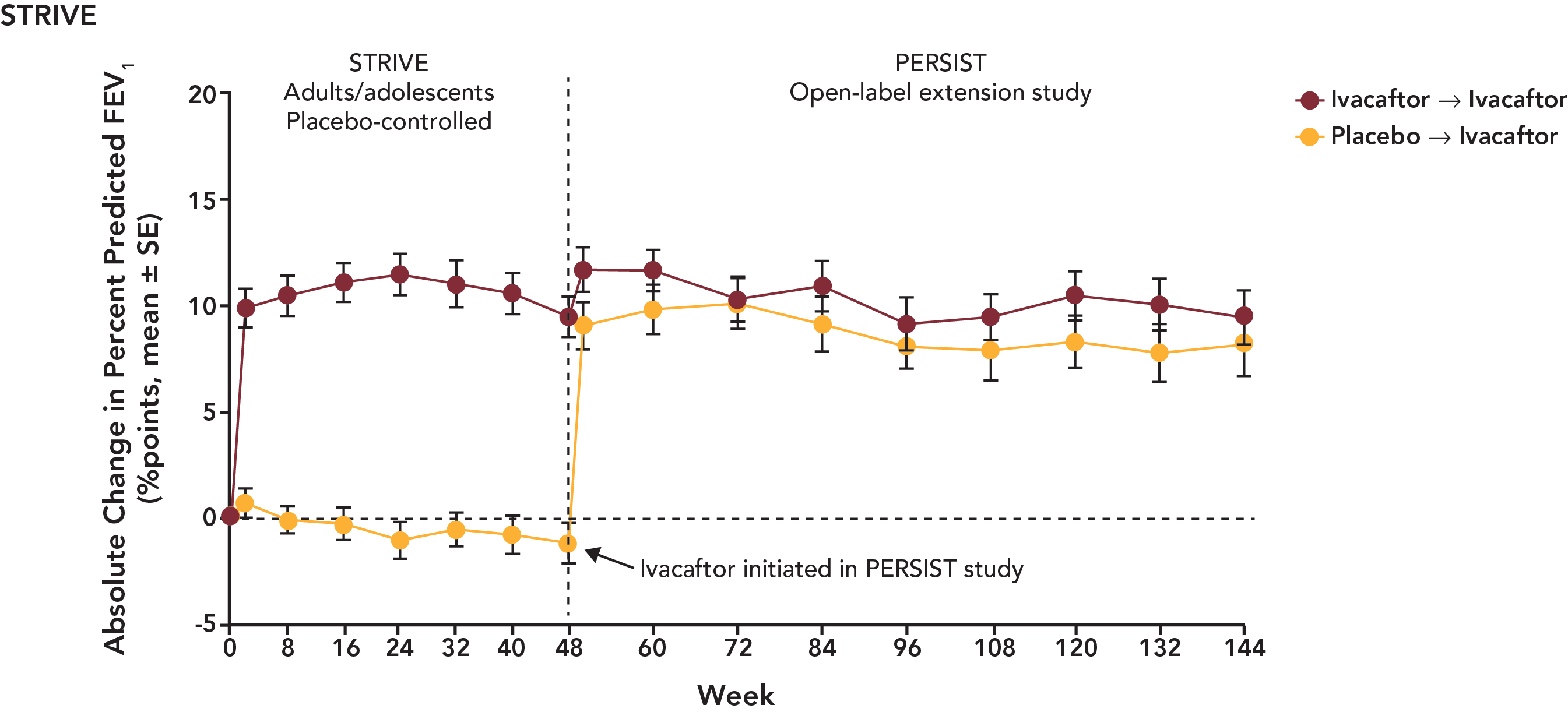
The PBAC noted the following data from PERSIST for STRIVE and ENVISION patients.
Updated PERSIST data for STRIVE patients are presented in the following figure:
Updated PERSIST data for ENVISION patients are presented in the following figure:
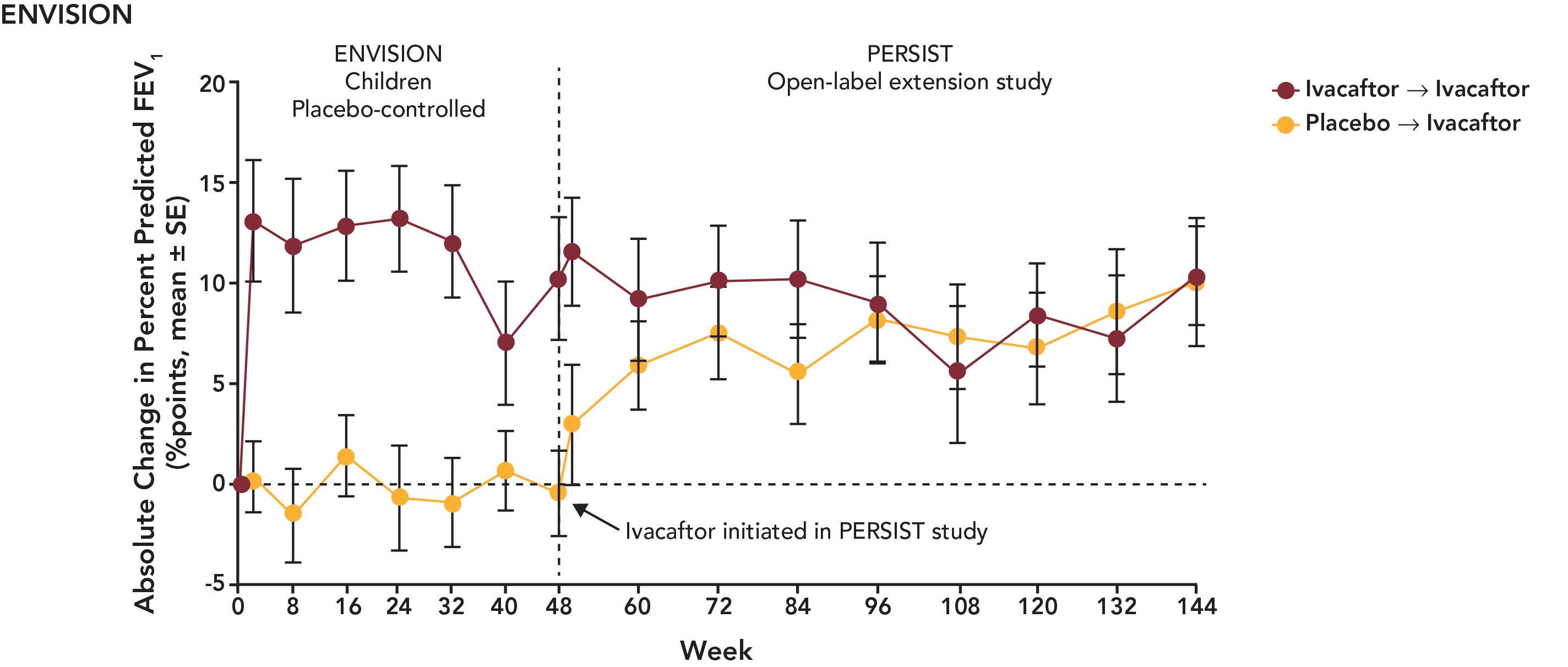
With regard to comparative effectiveness, the PBAC recalled that it accepted in July 2013 the claim of superiority of ivacaftor over best supportive care. This claim was supported in terms of the effect on the surrogate outcomes reported in the trials at 24 at 48 weeks, including % predicted FEV1, weight gain and quality of life. The PBAC noted that the effect of ivacaftor was maintained in the data presented from the PERSIST open label extension trial out to 144 weeks. The PBAC, as in July and November 2013, again recognised the potential clinical value of ivacaftor in the treatment of CF. The PBAC re-affirmed its view that at present, it remains that the short term follow-up period meant that there was no evidence directly from the trials that the change in FEV1 will translate into long term survival benefits.
The PBAC noted that the data on comparative harms remained unchanged from the July 2013 submission. The PBAC also noted that data for harms for patient in the BSC arm were not available at 144 weeks, as patients originally enrolled in the BSC arm were crossed over to receive ivacaftor once they entered the PERSIST extension study.
The PBAC noted that the proportion of patients with adverse events appeared to be steady between the initial STRIVE / ENVISION and PERSIST trials. Overall, the PBAC considered that the PERSIST data demonstrated a similar safety profile out to 144 weeks to that considered in July 2013. The PBAC did consider that there is some residual uncertainty due to the likely use of lifetime treatment with ivacaftor.
A summary of the comparative benefits and harms for ivacaftor versus BSC is presented in the table below.
Benefit/harm summary
|
Outcome |
N
|
Absolute change from baseline |
Increment |
|
|---|---|---|---|---|
| Ivacaftor | BSC | |||
|
Benefits |
||||
|
Percent Predicted FEV1 Score (ENVISION/PERSIST) |
||||
|
Week 48 |
51 |
+10.67 |
+0.68 |
9.99 (4.52,15.46) |
|
Week 144 |
|
+10.30 |
N/R |
N/A |
|
Percent Predicted FEV1 Score (STRIVE/PERSIST) |
||||
|
Week 48 |
161 |
+10.13 |
-0.37 |
10.50 (8.50,12.50) |
|
Week 144 |
|
+9.40 |
N/R |
N/A |
|
Harms (SAE presented in preference to AE, as almost all patients experience an AE) |
||||
|
Any SAE (to 48 weeks) |
213 |
22.9% |
37.5% |
-14.6% |
|
Any SAE (48 to 96 weeks) |
192 |
14.6% |
N/R |
N/A |
|
Any SAE (96 to 144 weeks) |
192 |
22.9% |
N/R |
N/A |
9. Clinical Claim
The clinical claim of superiority over BSC in terms of comparative effectiveness, and equivalent comparative safety, was unchanged since the July 2013 submission. The PBAC recalled it had already accepted these claims in the short term.
The PBAC noted the data from the PERSIST extension study and considered that the clinical claim was supported out to the 144 weeks of the study. The PBAC remained unconvinced that the clinical claim was supported beyond 144 weeks, noting that ivacaftor may be used as a lifelong treatment.
10. Economic Analysis
The model structure was the same as in the July 2013 submission. The base case ICER in the resubmission was more than $100,000 per QALY gained, compared with an ICER of more than $200,000 per QALY in the July 2013 submission.
The PBAC recalled from its July 2013 consideration that the price of ivacaftor was the principal driver in the cost effectiveness of ivacaftor
The PBAC considered that the reduction in the adherence rate, with no proportionate reduction in the modelled effectiveness of ivacaftor, favoured ivacaftor. The PBAC noted that the sponsor agreed in its pre-subcommittee response to use a higher adherence rate.
The PBAC noted the assumption of a reduction in mean dose of ivacaftor to account for patients that require a CYP3A inhibitor. The PBAC considered that this assumption was not adequately supported, and may favour ivacaftor.
In terms of pricing, the PBAC considered that ivacaftor would not be cost-effective on the basis proposed by the sponsor in its resubmission: namely, at the price proposed by the sponsor. The PBAC remained of the view, previously expressed in November 2013, that the cost-effectiveness of ivacaftor would be acceptable if the ICER was between $60,000 and $80,000 per QALY gained, which could not be achieved on the basis proposed in the sponsor’s resubmission.
The PBAC considered that, in the absence of a lower price, the cost-effectiveness of ivacaftor could also be improved under a “pay for performance” arrangement, because the PBAC was satisfied that ivacaftor offers, for some patients, a significant improvement in efficacy compared with BSC. The PBAC was of the view that ivacaftor would be acceptably cost effective for patients who respond to treatment to the same extent and duration as depicted in the clinical data.
The PBAC considered that cost-effectiveness of ivacaftor would be acceptable if a “pay for performance” arrangement of the nature described below were implemented, together with the other risk sharing measures also identified below:
- An agreement between the sponsor of ivacaftor and the Government to cap the maximum financial expenditure to the submission’s estimates with a 100% rebate thereafter.
- A “pay-for-performance” arrangement whereby the sponsor rebates to the Commonwealth 100% of the cost of treatment with ivacaftor received by patients who are subsequently assessed as not responding to treatment every 3 and 6 months. Response for that purpose should be defined as at least a 5% improvement in FEV1 after three months’ treatment, and at least 10% improvement after six months treatment. For patients aged six to 11 years that are able to show improvement in FEV1 but less than the threshold for PBS subsidy, the assessment of clinical response may also include a weight gain of 1.5kg at three months and/or 3kg at six months. Where a patient’s response to ivacaftor is affected by acute infective exacerbation at the time of diagnosis, a single month’s additional PBS subsidised treatment might be authorised before reassessment.
- Where a patient currently uses a moderate or strong CYP3A inhibitor known to influence the pharmacokinetics of ivacaftor which must trigger a dose adjustment as per the recommended dose in the Product Information (150mg once daily for moderate inhibitors, 150mg twice weekly for strong inhibitors), the subsidy only be paid for the adjusted dose, with any difference between what is being used in clinical practice versus recommended PI dose to be rebated by the sponsor.
- Commitment by the sponsor for ongoing funding for collection of data in all patients receiving PBS-funded ivacaftor in accordance with the views expressed by the PBAC in November 2013.
- Every 12 months of data to be provided to the PBAC for assessment and comparison between the clinical trial and assumptions in the economic analysis and real life clinical experience.
11. Estimated PBS Usage and Financial Implications
The likely number of patients per year was estimated in the submission to be less than 10,000 patients in Year 5, at an estimated net cost to the Commonwealth of between $30 million and $60 million in Year 5. The total estimated cost was between $100 million and $200 million over five years.
The PBAC recalled that in November 2013 it considered that cost effectiveness for ivacaftor would involve the cost of the initial 3 to 6 months’ treatment per patient being met by the sponsor, until the patient’s response to treatment is determined. The PBAC considered that limiting PBS subsidy only to responding patients is critical to the cost effectiveness of ivacaftor.
12. PBAC Outcome
The PBAC reiterated its previous recommendation for the PBS listing of ivacaftor as a Section 100 (Highly Specialised Drugs Program) benefit for treatment of cystic fibrosis (CF) in patients aged six years and older who have a G551D mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. The PBAC also recommended that access to the listing be by written Authority application, to be handled by the Complex Drugs area of the Department of Human Services.
Taking into account the conclusions expressed above in relation to the monitoring of sweat chloride, the PBAC considered that the restriction suggested in November 2013 needed to reflect that sweat chloride testing is just one of the eligibility and monitoring criteria, and it should not be used in isolation.
If the alternative approach noted above. where the PBAC considered cost effectiveness of ivacaftor would be acceptable if a “pay for performance arrangement together with other risk sharing measures were adopted, the restrictions that the PBAC would recommend would be as follows:
The eligibility for initial access to ivacaftor be determined on the following basis:
- Age greater than or equal to 6 years; and
- A sweat chloride value greater than or equal to 60mmol/L by quantitative pilocarpine iotophoresis or 2CF-causing mutations (all as documented in the patient’s medical record); and
- Presence of a G551D mutation of the CFTR gene; and
- Presence of chronic sinopulmonary disease or gastrointestinal/nutritional abnormalities; and
- FEV1 greater than or equal to 40% of predicted (as per the inclusion criteria in the clinical trials) measured in accredited pulmonary function laboratory, with documentation of no acute infective exacerbation at the time of testing.
With the initial Authority application, the baseline levels of the following must be provided:
- Sweat chloride
- Stable FEV1 measurement, expressed as percent of predicted
- CYP3A inhibitors and inducers – medication history and anticipated use; and
- Baseline Cystic Fibrosis Questionnaire – Revised (CFQ-R) score
- Patient weight
Patients must sign a consent form that “no response” will result in PBS subsidy being discontinued.
The PBAC recommended that at the initial Authority application, a maximum of three months’ supply be authorised. For a patient that is able to demonstrate a response to treatment, a further three months’ supply may be authorised at that point, with a further clinical assessment after a total of six months’ treatment. For a patient able to demonstrate continued response to treatment, ongoing treatment may be authorised with clinical reassessment at six month intervals.
The PBAC recommended that the patient must be assessed at a CF clinic or centre which is under the control of specialist respiratory physicians with experience and expertise in the management of CF. If attendance at such a unit is not possible because of geographical isolation, management (including prescribing) may be in consultation with such a unit.
The PBAC recommended that measurement of lung function is to be conducted by independent (other than the treating doctor) experienced personnel at an established lung function testing laboratory, unless this is not possible because of geographical isolation.
The PBAC recommended the following criteria for eligibility at the three-month clinical assessment, in order to access a further three months of treatment:
- Percent predicted FEV1
o An improvement of at least 5% from baseline.
- Weight gain
o Where a patient aged from 6 to 11 years is able to demonstrate an improvement in FEV1 but not sufficient to reach 5%, the patient may be judged to have responded to treatment if they are also able to demonstrate a gain in weight of at least 1.5kg after the first 3 months of treatment with ivacaftor.
The PBAC recommended the following criteria for eligibility at the six-month clinical assessment, in order to access ongoing treatment:
- Percent predicted FEV1
o An improvement of at least 10% from baseline.
- Weight gain
o Where a patient aged from 6 to 11 years is able to demonstrate an improvement in FEV1 of between 5% and 10%, the patient may be judged to have responded to treatment if they are also able to demonstrate a gain in weight of at least 3kg after 6 months of treatment with ivacaftor.
The PBAC recommended where a patient’s ability to demonstrate a response to ivacaftor is affected by an acute infective exacerbation at the time of assessment, one additional month’s supply may be authorised, with the patient to be reassessed before the end of the additional month’s treatment. Prescribers will be required to provide written evidence that the patient’s exacerbation is due to acute infection.
All the de-identified information collected through the written authorities system should be recorded in the Australian Cystic Fibrosis Database Registry.
Noting that ivacaftor was used in the trials as an add-on to standard CF treatment, the PBAC recommended that ivacaftor be used in combination with other CF treatments for the purpose of PBS subsidy. The PBAC recommended that prescribers be required to provide details of concomitant medications being used by the patient, in line with the clinical trials available for ivacaftor. Similarly, the PBAC noted that the exclusion criteria applied in ivacaftor trials and advised that these criteria should also be used in clinical practice.
Where a patient currently uses a moderate or strong CYP3A inhibitor known to influence the pharmacokinetics of ivacaftor, the PBAC recommended that the appropriately adjusted supply for one month’s supply per prescription at the recommended dose in the Product Information (150mg once daily for moderate inhibitors, 150mg twice weekly for strong inhibitors) a subsidy will only paid for the adjusted dose, with any difference to be rebated by the sponsor.
The PBAC recommended that the use of CYP3A inducers be an exclusion criterion for PBS access, noting the potential for compromised treatment effect at extremely high cost to the Commonwealth.
The PBAC acknowledged that if that alternative approach were adopted, that it would involve significant administrative and compliance measures on prescribers, patients and the Government, and inconvenience for each. However, the PBAC has suggested those measures in an effort to identify a means of achieving the PBS listing of the drug on a basis which is cost-effective, if the sponsor is not willing to offer the drug to the Government at a price that achieves cost-effectiveness, and that the Government considers acceptable.
The PBAC considered that it would be desirable that any data collected pursuant to any arrangements agreed with the sponsor be placed in the public domain to develop knowledge of CF for government, industry and academia alike.
The PBAC also considered that it would be desirable that the utilisation and cost effectiveness of ivacaftor under any pay for performance arrangement be reassessed once 12 months of data are available post listing.
The PBAC recommended that the Safety Net 20 Day Rule should apply.
Recommendation:
Add new item:
Restriction to be finalised
13. Context for Decision
The PBAC helps decide whether and, if so, how medicines should be subsidised in Australia. It considers submissions in this context. A PBAC decision not to recommend listing or not to recommend changing a listing does not represent a final PBAC view about the merits of the medicine. A company can resubmit to the PBAC or seek independent review of the PBAC decision.
14. Sponsor’s Comment
The sponsor has no comment.



