Ranibizumab, solution for intravitreal injection, 3.0 mg/0.3 mL, Lucentis®, March 2007
Public summary document for Ranibizumab, solution for intravitreal injection, 3.0 mg/0.3 mL, Lucentis®, March 2007
Page last updated: 29 June 2007
Public Summary Document
Product: Ranibizumab, solution for intravitreal
injection, 3.0 mg/0.3 mL, Lucentis®
Sponsor: Novartis Pharmaceuticals Australia Pty
Ltd
Date of PBAC Consideration: March 2007
1. Purpose of Application
The submission sought a Section 85 Authority Required listing for
neovascular age-related macular degeneration (AMD).
2. Background
The PBAC had not previously considered this drug.
3. Registration Status
Lucentis was registered by the TGA on 27 February 2007 for the
treatment of neovascular (“wet”) age-related macular
degeneration (AMD). Lucentis 0.5 mg or 0.3 mg is recommended to be
administered by intravitreal injection once a month.
4. Listing Requested and PBAC’s View
The following wording of the restriction was proposed for the
“prn” (when required) dosing regimen:
Initial treatment
Active neovascular (wet) age-related macular degeneration. The
eye(s) being treated must be specified in the authority
application.
Continuation treatment
Neovascular (wet) age-related macular degeneration (AMD), in
patients previously treated with ranibizumab with evidence of
continued disease activity as defined by a loss of 5 letters of
visual acuity (ETDRS or one Snellen line equivalent) and/or
evidence of leakage of fluid, haemorrhage or lesion growth.
The visual acuity loss must be relative to the best visual acuity
achieved on ranibizumab therapy. The most recent best visual acuity
measure must be within the past 12 months.
Re-treatment should not be given more frequently than once every
month.
The following wording of the restriction was proposed for the
“monthly” dosing regimen:
Active neovascular (wet) age-related macular degeneration. The
eye(s) being treated must be specified in the authority
application.
See Recommendations and Reasons for PBAC’s
view
5. Clinical Place for the Proposed Therapy
AMD is a progressive disease of the retina that results in loss of
central vision, leaving only peripheral vision intact. The
exudative or neovascular (wet) form of AMD involves the breaching
of a functional retinal barrier and growth of abnormal blood
vessels into the central part of the retina. Ranibizumab is a new
treatment for exudative macular degeneration.
6. Comparator
The submission nominated two comparators. Consistent with the
current available reimbursed treatment for subfoveal neovascular
AMD; photodynamic therapy with verteporfin (PDT-V) is the main
comparator for ranibizumab when used to treat patients with
predominantly classic lesions and standard care is the main
comparator for ranibizumab when used to treat patients with occult
or minimally classic lesions.
7. Clinical Trials
The submission presented the following three randomised comparative trials:
- ANCHOR trial, comparing ranibizumab (administered monthly) with PDT-V in patients with predominantly classic lesions;
- MARINA trial, comparing ranibizumab (administered monthly) with placebo in patients with minimally classic and occult CNV lesions; and
- PIER trial, comparing ranibizumab (administered for three consecutive months, then administered every three months) with placebo in patients with predominantly and minimally classic, and occult CNV lesions.
Two of these studies had been published at the time of the submission, as follows:
| Trial/First author | Protocol title/Publication title | Publication citation |
|---|---|---|
| ANCHOR/Brown | Ranibizumab vs verteporfin for neovascular age-related macular degeneration (report of trial results at 12 months) | Brown et al. N Engl J Med 2006;355:1432-44 |
| MARINA/Rosenfeld | Ranibizumab for neovascular age-related macular degeneration (report of trial results at 12 and 24 months) | Rosenfeld et al. N Engl J Med 2006; 355:1419-31 |
8. Results of Trials
The results of the key trials are summarised in the tables below. Some trial results/numbers
in this PSD are taken from cited publications and may vary slightly from numbers considered
by the PBAC which were taken from the sponsor’s internal reports.
Proportion of patients losing fewer than 15 letters in visual acuity compared with
baseline at 12 and 24 months, based on assessment at a starting test distance of 2
metres
| Trial | Sham injection | PDT-V | RAN 0.3mg | RAN 0.5mg | ARD§ (95% CI) | |
|---|---|---|---|---|---|---|
| Results at 12 months | ||||||
| ANCHOR a | 92/143 (64.3%) | 132/140 (94.3%) | 134/139 (96.4%) | RAN 0.3 vs PDT-V 30.1% (21.8%, 38.5%) | RAN 0.5 vs PDT-V 31.5% (24.5%, 40.6%) | |
| MARINA a | 148/238 (62.2%) | 225/238 (94.5%) | 227/240 (94.6%) | RAN 0.3 vs Sham 31.9% (25.4%, 38.5%) | RAN 0.5 vs Sham 32.0% (25.5%, 38.6%) | |
| PIER | NR | NR | NR | NR | NR | |
| Results at 24 months | ||||||
| MARINA | 126/238 (52.9%) | 219/238 (92%) | 216/240 (90%) | RAN 0.3 vs Sham 38.7% (31.7%, 45.7%) | RAN 0.5 vs Sham 36.6% (29.4%, 43.7%) | |
* From the Cochran chi square tests adjusted for the strata
§ Weighted estimates adjusting for the strata by using Cochran-Mantel-Haenszel weights
a primary outcome of the trials
The data suggested that ranibizumab reduced visual loss at 12 and 24 months in monthly
dosing regimen compared with both PDT-V and with placebo. Ranibizumab had a similar
effect in predominantly classic CNV-AMD (at 12 months) and in combined minimally classic
and occult CNV-AMD (at 12 and 24 months). The 0.3 mg and 0.5 mg doses appeared to
be similar in efficacy at the 24-month timepoint.
Proportion of patients gaining ?15 letters in visual acuity compared with baseline
at 12 and 24 months, based on assessment at a starting test distance of 2 metres
| Trial | Sham injection | PDT-V | RAN 0.3mg | RAN 0.5mg | ARD§ (95% CI) | |
|---|---|---|---|---|---|---|
| 12 months | ||||||
| ANCHOR | 8/143 (5.6%) | 50/140 (35.7%) | 56/140 (40.3%) | RAN 0.3 vs PDT-V 30.1% (21.4%, 38.8%) | RAN 0.5 vs PDT-V 34.9% (25.9%, 43.8%) | |
| MARINA | 11/238 (5.0 %) | 59/238 (24.8%) | 81/240 (33.8%) | RAN 0.3 vs Sham 19.9% (13.8%, 25.9%) | RAN 0.5 vs Sham 28.7% (22.3%, 35.0%) | |
| PIER | NR | NR | NR | NR | NR | |
| 24 months | ||||||
| MARINA | 9/238 (3.8%) | 62/238 (26.1%) | 80/240 (33.3%) | RAN 0.3 vs Sham 21.9% (16.0%, 27.9%) | RAN 0.5 vs Sham 29.2% (22.9%, 35.4%) | |
The data above suggested that monthly ranibizumab led to improvements in vision in
about 20-30% of patients with all types of CNV-AMD at 12 and 24 months compared with
PDT-V and with PBO. The 0.5 mg dose generally appeared to be more effective than the
0.3 mg dose.
Although statistically significant differences were observed in proportions of patients
gaining ?15 letters in visual acuity between ranibizumab-treated and control subjects
in the trials where ranibizumab was administered monthly, there was no significant
difference in the proportion of patients gaining ?15 letters in visual acuity when
ranibizumab was administered in three-monthly doses followed by administration every
three months compared with placebo in patients with any CNV lesion type (results from
PIER). This suggested that treatment on a monthly basis may be associated with improved
outcomes compared with treatment every 3 months.
PIER Mean Change from Baseline over Time in Visual Acuity
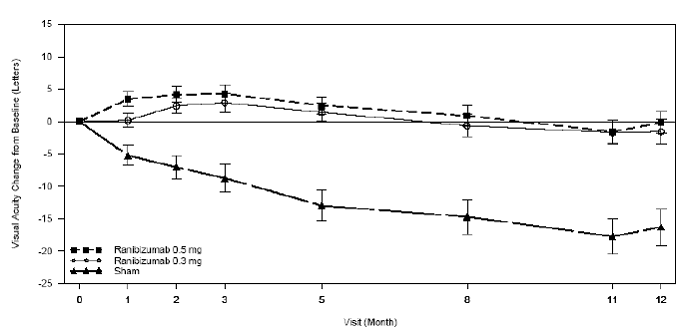
Statistically significant differences in effectiveness were generally observed in
the results from the MARINA trial between patients treated with ranibizumab 0.3 mg
compared with patients treated with ranibizumab 0.5 mg, with results favouring ranibizumab
0.5 mg.
Although the same trend was observed in the other trials, differences between doses
did not reach statistical significance.
In relation to the comparison of monthly ranibizumab versus PDT-V (ANCHOR trial),
the incidence of ocular adverse events related to the study drug was higher in the
ranibizumab treatment arms compared with the PDT-V arm. The ocular adverse events
occurring more frequently in the ranibizumab-treated arms than PDT-V arm include conjunctival
haemorrhage, increased intraocular pressure, eye pain, iritis, vitritis and vitreous
floaters.
9. Clinical Claim
Ranibizumab has significant advantages in effectiveness over PDT-V
but is associated with greater toxicity in patients with
predominantly classic CNV lesions in patients with visual acuity
≥20/200; ranibizumab has significant advantages in effectiveness
over placebo but is associated with greater toxicity in patients
with minimally classic and occult CNV lesions. The PBAC considered
the claim reasonable.
10. Economic Analysis
The submission presented a series of preliminary (trial-based)
economic evaluations based on the results from the ANCHOR, MARINA,
and PIER trials. The choice of the cost-effectiveness approach was
valid. The resources included were drug costs, costs of
administering ranibizumab by intravitreal injection, costs of
administering PDT-V (infusing verteporfin and subsequent laser
irradiation of the macula), and costs of monitoring (follow-up
consultation, diagnostic tests and ophthalmic imaging). The overall
comparative costs and outcomes for each alternative and the
incremental costs and outcomes are summarised below.
Based on the results of the ANCHOR trial the incremental cost/extra
patient losing < 15 letters at the 0.5 mg ranibizumab dose over
12 months was between $45,000 and $75,000.
Based on the results of the MARINA trial the incremental cost/extra
patient losing < 15 letters over 24 months was between $105,000
and $200,000.
Based on the PIER trial the incremental cost/extra patients losing
< 15 letters over 12 months was between $15,000 and
$45,000.
The submission presented a series of modelled economic evaluations.
The choice of the cost-utility approach was appropriate. The PBAC
noted the 5-year model incorporated the effects of a risk sharing
agreement.
In patients with predominately classic CNV lesion the incremental
cost-effectiveness ratio/extra QALY gained for ranibizumab 0.5 mg
monthly was between $15,000 and $45,000.
In patients with predominately classic CNV lesions the incremental
cost-effectiveness ratio/extra QALY gained for ranibizumab 0.5 mg
monthly for 3 months then every 2 months was between $15,000 and
$45,000.
In patients with minimally classic CNV lesions the incremental
cost/extra QALY gained for ranibizumab 0.5 mg monthly was between
$$45,000 and $75,000.
In patients with minimally classic CNV lesions the incremental
cost/extra QALY gained for ranibizumab 0.5 monthly for 3 months
then every 2 months was between $105,000 and $200,000.
In patients with occult CNV lesions, the incremental cost/extra
QALY gained for ranibizumab 0.5 mg monthly was between $45,000 and
$75,000.
In patients with occult CNV lesions the incremental cost/extra QALY
gained for ranibizumab 0.5 monthly for 3 months then every 2 months
was between $105,000 and $200,000.
The weighted average cost-effectiveness of ranibizumab 0.5 monthly
for all lesions types was between $15,000 and $45,000.
The weighted average cost-effectiveness of ranibizumab 0.5 monthly
for 3 months then every 2 months $45,000 and $75,000.
11. Estimated PBS Usage and Financial Implications
The predicted net cost to government was estimated to be > $100
million per year.
12. Recommendation and Reasons
The PBAC recommended listing for the treatment of subfoveal
choroidal neovascularisation (CNV) due to age related macular
degeneration on a cost-effectiveness basis against verteporfin with
PDT in predominantly classic disease, and against placebo in
minimally classic or occult disease. Listing was recommended at the
price proposed in the submission on the basis of an average
incremental cost per extra quality adjusted life year (QALY) gained
across all lesion types of between $15,000 and $45,000 on the basis
of the arrangement proposed by the sponsor.
The PBAC did not agree to the sponsor’s proposed
“prn” (when necessary) dosing regimen, noting that (a)
it is not consistent with the TGA recommended dosing regimen and
(b) there is currently no direct clinical trial evidence supporting
the efficacy of treatment using the regimen proposed and some
clinical trial evidence from the PIER trial which suggests it may
be associated with worse outcomes than monthly dosing. Of the three
key trials submitted in support of listing, two (ANCHOR and MARINA)
used a monthly dosing schedule and one (PIER) used a less frequent
dosing schedule, albeit different to the sponsor’s proposed
“prn” schedule. A comparison across the results of the
three trials suggests that injections given on a monthly basis may
be associated with improved outcomes compared with injections given
every 3 months. The PBAC considered it was important that an
appropriate Quality Use of Medicines (QUM) program be undertaken to
ensure that clinicians are aware of the most effective dosing
schedule for ranibizumab.
The PBAC further recommended that prescribing under the PBS be
restricted to ophthalmologists as the sole CNV therapy subsidised
at any one time.
The PBAC indicated concern about the large amount of wastage and
suggested that the sponsor investigate a smaller pack size. The
PBAC also indicated concern about the storage of this product and
the need to maintain the cold-chain. This issue is of particular
concern for the period between dispensing and administration and
the PBAC requested the sponsor implement QUM measures to minimise
the chance of the cold-chain being interrupted at this point.
Recommendation
RANIBIZUMAB, solution for intravitreal injection, 3.0 mg/0.3
mL,
Authority Required
Initial treatment by an ophthalmologist, as the sole subsidised
therapy, of subfoveal choroidal neovascularisation (CNV) due to age
related macular degeneration (AMD), as diagnosed by fluorescein
angiography.
Authority approvals will be administered by the PBS and Specialised
Drugs Branch of Medicare Australia.
The first authority application for each eye must be made in
writing, and must include:
a) completed authority prescription form;
b) completed Subfoveal Choroidal Neovascularisation (CNV) –
PBS Supporting Information Form [www.medicare.gov.au]; and
c) a copy of the fluorescein angiogram.
Written applications for authority to prescribe ranibizumab should
be forwarded to:
Medicare Australia
Prior Written Approval of Specialised Drugs
Reply Paid 9826
GPO Box 9826
HOBART TAS 7001
Alternatively, the first authority application may be faxed to
Medicare Australia on (03) 6215 5474 (hours of operation 8 a.m. to
5 p.m. EST Monday to Friday). Medicare Australia will then contact
the prescriber by telephone. The original documentation must be
posted to the above address after approval has been gained.
Max Quantity: 1
Number of Repeats: 0
Authority Required
Continuing treatment by an ophthalmologist, as the sole subsidised
therapy, of subfoveal choroidal neovascularisation (CNV) due to age
related macular degeneration (AMD) where the patient has previously
been granted an authority prescription for the same eye.
Authority approvals will be administered by the PBS and Specialised
Drugs Branch of Medicare Australia. Authority applications for
continuing treatment in the same eye may be made by telephone on
1800 700 270 (hours of operation 8 a.m. to 5 p.m. EST Monday to
Friday).
Max Quantity: 1
Number of Repeats: 1
13. Context for Decision
The PBAC helps decide whether and, if so, how medicines should be
subsidised in Australia. It considers submissions in this context.
A PBAC decision not to recommend listing or not to recommend
changing a listing does not represent a final PBAC view about the
merits of the medicine. A company can resubmit to the PBAC or seek
independent review of the PBAC decision.
14. Sponsor’s Comment
The sponsor chose not to make a comment.




