Etoricoxib, tablets, 60 mg, Arcoxia, July 2007
Public summary document for Etoricoxib, tablets, 60 mg, Arcoxia, July 2007
Page last updated: 26 October 2007
PDF version of this page (PDF 42KB)
Public Summary Document
Product: Etoricoxib, tablets, 60 mg, Arcoxia
Sponsor: Merck Sharp & Dohme (Australia) Pty Limited
Date of PBAC Consideration: July 2007
1. Purpose of Application
The submission sought a section 85 restricted benefit listing for etoricoxib 60 mg tablets for the symptomatic treatment osteoarthritis.
2. Background
At the March 2004 meeting, the PBAC rejected a submission for a restricted benefit listing for etoricoxib for the symptomatic treatment of osteoarthritis (60 mg) and rheumatoid arthritis (90 mg) on the grounds of uncertainty over the comparative safety claim.
3. Registration Status
Etoricoxib is TGA registered for:
- Symptomatic treatment of the signs and symptoms of osteoarthritis (OA)
- Treatment of acute gouty arthritis
- Treatment of acute pain, including that related to primary dysmenorrhea and minor dental procedures.
4. Listing Requested and PBAC’s View
NOTE:
The use of etoricoxib for the treatment of the following conditions is not subsidised
through the PBS:
Acute pain;
Soft tissue injury;
Arthrosis without inflammatory component.
Restricted benefit:
Symptomatic treatment of osteoarthritis
Given the maximum dose for osteoarthritis is 60 mg once daily, the PBAC considered
that a NOTE prohibiting increased maximum quantities and repeats would be appropriate,
if listing were to proceed.
5. Clinical Place for the Proposed Therapy
Etoricoxib would provide an alternative selective cyclooxygenase-2 (Cox-2) inhibitor for treatment of patients with symptomatic osteoarthritis.
6. Comparator
The submission nominated celecoxib as the main comparator and lumiracoxib as the minor
comparator. The PBAC considered that this was appropriate given the requested restriction.
Subsequent to the PBAC consideration of etoricoxib, the TGA registration of lumiracoxib
was cancelled. In view of this, information relating to the comparision of etoricoxib
and lumiracoxib has not been reported in this Public Summary Document.
For consistency with previous PBAC considerations of osteoarthritis drugs (celecoxib,
rofecoxib and meloxicam) a comparison with traditional non-steroidal anti-inflammatory
drugs (tNSAIDs) was also appropriate for the listing of a new COX-2 inhibitor.
7. Clinical Trials
New long-term outcomes studies (EDGE 1, EDGE 2 and the MEDAL study) have been conducted
in patients with OA and RA. Results from these studies provided additional data on
the gastrointestinal and cardiovascular profile of etoricoxib for this resubmission.
The basis of the re-submission was:
- one randomised trial (P007) comparing the efficacy of etoricoxib 30 mg to etoricoxib 60 mg and placebo;
- two direct randomised trials (P076/P077) comparing the efficacy of etoricoxib 30 mg to celecoxib 200 mg;
- three indirect safety meta-analyses of supplementary randomised trials indirectly comparing the gastrointestinal, cardiovascular and hypertension safety profiles of etoricoxib with celecoxib. This was done using, variously, tNSAIDs as a group, individual NSAIDs (ie naproxen) or placebo as the common reference. The Multinational Etoricoxib and Diclofenac Arthritis Long-term (MEDAL) clinical study program consists of 3 individual, randomised, double-blind clinical studies: EDGE, EDGE II and MEDAL. The MEDAL program compared etoricoxib 60 mg/90 mg to diclofenac 150 mg (the CLASS trial compared celecoxib 800 mg to diclofenac 150 mg and to ibuprofen 2400 mg. Across the MEDAL program, 34,701 patients had osteoarthritis (72%) and 9,787 (28%) had rheumatoid arthritis. The number of patients receiving etoricoxib in the program was 17,412.
The key trials had been published at the time of submission as follows:
|
Trial/First author |
Protocol title/Publication title |
Publication citation |
|---|---|---|
|
Etoricoxib |
||
|
P076/P077 |
Efficacy and safety of etoricoxib and celecoxib 200 mg in the treatment of OA in two identically designed, randomised, placebo controlled, non-inferiority studies. |
Rheumatology;46:pp496-507 |
|
P007 |
Etoricoxib in the treatment of OA over 52 weeks. Results of a randomized, dose-ranging trial of etoricoxib in patients with OA. |
BMC Musculoskeletal Disorders; 6(58), p2489 |
|
P066 MEDAL Program (which comprised EDGE 1, EDGE 2 and the MEDAL study) |
Cardiovascular outcomes with etoricoxib and diclofenac in patients with OA and RA arthritis in the Multinational Etoricoxib and Diclofenac Arthritis Long-term (MEDAL) programme. |
Lancet. 368(9549): pp1771-81 |
|
CLASS |
Celecoxib versus NSAIDs |
JAMA; 284(10):pp1247-55 |
|
TARGET |
Lumiracoxib versus NSAIDs |
Lancet.; 364(9435):pp665-74 |
The PBAC considered that the use of diclofenac as the comparator is a major deficiency in the interpretation of the MEDAL program data. Even though diclofenac is termed a “traditional” NSAID, there is significant scientific evidence to indicate that it is a relatively COX-2 selective drug with a similar degree of COX-2 selectivity to celecoxib in some studies.
8. Results of Trials
Comparative effectiveness
The results of trial 007, in which the efficacy of etoricoxib 30 mg or 60 mg was compared
with placebo, demonstrated a statistically significant and a clinically important
reduction in the primary outcome of pain compared to placebo.
Reduction in pain, in patients treated with 60 mg etoricoxib, was significantly greater,
although not to a clinically important extent, compared to patients treated with 30
mg etoricoxib
Based on the results of trial P076/077, which compared the efficacy of etoricoxib
30 mg with celecoxib 200 mg, the PBAC agreed that treatment with etoricoxib 30 mg
appeared to be no worse than treatment with celecoxib 200 mg in terms of reducing
pain, improving function and patient global assessment of disease status (PGADS).
Comparative toxicity
Gastrointestinal
The submission presented results of the GI safety comparisons in terms of complicated
perforations, ulcers and bleeds (PUBs)/perforations, obstructions and bleeds (POBs)
between the large etoricoxib trial (the MEDAL program) and celecoxib trial (CLASS).
Traditional NSAIDs were again the “common” reference.
Gastrointestinal events across the CLASS study and MEDAL program trials
|
Trial |
Treatment group |
n/N (%) |
ARD |
RR |
p-valuea |
|---|---|---|---|---|---|
|
All patients PUBs† |
|||||
|
CLASS b |
Celecoxib 400 mg BD |
1.08% |
-0.48% |
0.69 |
0.0609 |
|
|
Traditional NSAIDs |
1.56% |
|
|
|
|
MEDAL program |
Etoricoxib |
1.01% |
-0.41% |
0.71 |
0.0005 |
|
|
Traditional |
1.42% |
|
|
|
|
All patients complicated PUBs or POBs‡ |
|||||
|
CLASS b |
Celecoxib 400 mg BD |
17/3987 |
-0.10% |
0.81 |
0.5124 |
|
|
Traditional NSAIDs |
21/3981 |
|
|
|
|
MEDAL program |
Etoricoxib |
78/17,412 |
-0.03% |
0.94 |
0.7174 |
|
|
Traditional |
82/17,289 |
|
|
|
a x 2 p-value; b Data from entire study period;† defined as CSUGIES/GDU in CLASS ‡ defined as CSUGIEs
in CLASS and clinically significant bleeding); ARD = absolute risk difference; RR
= relative risk.
The PBAC was of opinion that etoricoxib and celecoxib both demonstrated a statistically
significant reduction in overall peptic ulcer and bleeds (PUBs) compared to traditional
NSAIDs (tNSAIDs). There was a trend toward reduced rate of POB/complicated PUBs for
both celecoxib in the CLASS trial and etoricoxib in the MEDAL Program versus tNSAIDs
but this trend did not reach statistical significance. However, the PBAC considered
that the data sets do not provide a scientifically robust basis to draw conclusive
assessments in regards to the comparison of the gastrointestinal safety profiles of
etoricoxib and celecoxib as shown by the statistically significant difference in the
rate of gastrointestinal events (PUBs) between the tNSAIDs. Although the PBAC has
previously accepted that complicated PUBs are equivalent to POBs, the definition of
these outcomes varied between trials.
Thus, although there was considerable uncertainty in the comparison of GI safety between
the COX-2 inhibitors, the PBAC accepted, on balance and particularly owing to the
lack of a common reference tNSAID as a comparator, that it is likely that etoricoxib
is similar in GI safety compared with celecoxib.
Cardiovascular
There was no difference between diclofenac 150 mg and etoricoxib (60 mg and 90 mg)
in terms of cardiovascular events shown in the MEDAL Program. (see figures below).
Medal Program – serious cardiovascular events: diclofenac vs etoricoxib
Thrombotic events
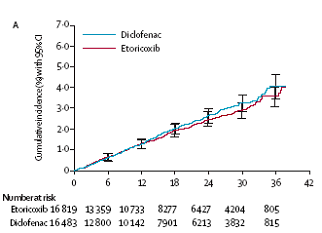
Arterial thrombotic events
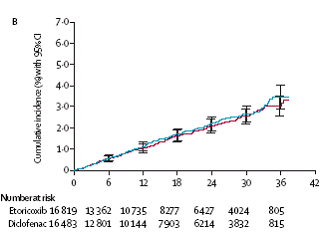
APTC events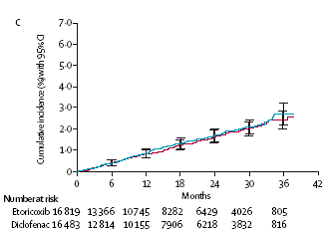
As for gastrointestinal safety, for cardiovascular (CV) safety profiles, the PBAC
considered that the data sets do not provide a scientifically robust basis to draw
a conclusive assessment in regards to the comparative safety of etoricoxib and celecoxib
beyond the assessments of either COX-2 selective NSAID compared with traditional NSAIDs.
However, the selection of diclofenac as a comparator in the MEDAL program is an issue
of concern because the results suggest that the CV profile of etoricoxib is non-inferior
to that of diclofenac, a traditional NSAID with similar COX-2 selectivity as celecoxib
and considered to have the worst CV risk among traditional NSAIDs.
The PBAC interpreted the results of the indirect comparison of etoricoxib versus celecoxib
to indicate that there was an increased rate of high blood pressure among users of
etoricoxib compared to users of celecoxib. The PBAC considered that the most at-risk
older patients who, by virtue of their age, were more likely to suffer from hypertension
would be the patient group most likely to be treated with etoricoxib on the PBS. Thus,
any increase in blood pressure, whether readily treatable or not, was of concern.
The PBAC also noted the higher rate of discontinuations due to ‘hypertension related
adverse effects’ of etoricoxib 60 mg compared with diclofenac 150 mg in the MEDAL
program.
9. Clinical Claim
The re-submission described etoricoxib as being no worse than celecoxib in terms of
effectiveness and safety.
The PBAC accepted this claim in terms of effectiveness, but considered that the claim
for similar safety had not been demonstrated because etoricoxib is more likely to
cause an increase in blood pressure.
10. Economic Analysis
The resubmission presented a cost minimisation analysis. As the PBAC did not accept the submission’s clinical claim, this analysis was not considered relevant.
11. Estimated PBS Usage and Financial Implications
The likely number of packs dispensed/year was estimated to be > 200,000 in Year 5.
The financial cost/year to the PBS was estimated to be < $10 million per year in Year
5.
12. Recommendation and Reasons
After taking into account all of the considerations described earlier in this Public Summary Document the PBAC decided to reject the submission on the basis of uncertain comparative safety in terms of hypertension.
13. Context for Decision
The PBAC helps decide whether and, if so, how medicines should be subsidised in Australia. It considers submissions in this context. A PBAC decision not to recommend listing or not to recommend changing a listing does not represent a final PBAC view about the merits of the medicine. A company can resubmit to the PBAC or seek independent review of the PBAC decision.
14. Sponsor’s Comment
The sponsor does not agree with the PBAC's interpretation of the indirect comparisons
of etoricoxib to celecoxib with respect to the rate of high blood pressure.
The majority of the data included in the indirect safety analysis was for patients
taking
120 mg, 90 mg and 60 mg doses of etoricoxib. The sponsor is seeking registration of
a 30 mg dose for osteoarthritis and looks forward to addressing the PBAC's concerns
by seeking additional data on the blood pressure profile of the 30 mg dose of etoricoxib
as the clinical effect of the lower dose may be different.




