Methylphenidate hydrochloride, extended release capsules, 20 mg, 30 mg and 40 mg, Ritalin LA®, July 2007
Public summary document for Methylphenidate hydrochloride, extended release capsules, 20 mg, 30 mg and 40 mg, Ritalin LA®, July 2007
Page last updated: 6 March 2024
PDF version of this page (PDF 269KB)
Public Summary Document
Product: Methylphenidate hydrochloride, extended release capsules, 20 mg, 30 mg and
40 mg, Ritalin LA
Sponsor: Novartis Pharmaceuticals Australia Pty Ltd
Date of PBAC Consideration: July 2007
1. Purpose of Application
The submission requested a Section 85 authority required listing of methylphenidate hydrochloride extended release capsules (Ritalin LA) for the treatment of attention deficit hyperactivity disorder (ADHD) in children and adolescents (6–18 years).
2. Background
The new strengths and formulation of the drug have not previously been considered by the PBAC.
3. Registration Status
Ritalin LA capsules were TGA approved for marketing on 15 August 2002 for the treatment of ADHD. Strengths: 20 mg, 30 mg and 40 mg.
4. Listing Requested and PBAC’s View
Authority required
Use in attention deficit hyperactive disorder in children and adolescents aged 6-18
years who have demonstrated a response to immediate release methylphenidate hydrochloride
with no emergence of serious adverse events, and who require coverage beyond that
provided by immediate release methylphenidate hydrochloride preparations.
See Recommendation and Reasons for PBAC’s view.
5. Clinical Place for the Proposed Therapy
An extended release formulation of methylphenidate hydrochloride, allowing for single daily morning dosing, will assist in compliance with therapy for ADHD.
6. Comparator
Appropriately, the submission nominated Concerta as the main comparator. Concerta
is an alternative extended release methylphenidate product.
The PBAC considered that immediate release methylphenidate is an appropriate additional
comparator and that a clinical comparison of Ritalin LA against twice daily immediate
release methylphenidate would have been informative.
7. Clinical Trials
The submission presented two randomised trials directly comparing single doses of
different strengths of Ritalin LA and Concerta. These were randomised crossover trials
in subjects who were already stabilised on methylphenidate-immediate release (MPH-IR)
twice daily and who continued to take this between the test doses, other than during
a 24 hour washout period prior to the test dose when subjects received no methylphenidate.
The submission also included two non-randomised crossover supportive trials comparing
Ritalin LA with MPH-IR and data from three pharmacokinetic studies comparing blood
concentration time curves with Ritalin LA, Concerta and MPH-IR.
The trials which had been published at the time of submission are as follows:
|
Trial/First author |
Protocol title/Publication title |
Publication citation |
|---|---|---|
|
Direct randomised trials: Ritalin LA versus Concerta |
||
|
US05 |
Comparative efficacy of two once daily methylphenidate formulations (Ritalin LA and Concerta) and placebo in children with attention deficit hyperactivity disorder across the school day. |
Pediatric Drugs 2003; 5(8):545-555 |
|
US07 |
Efficacy of two long-acting methylphenidate formulations in children with attention-deficit/hyperactivity disorder in a laboratory classroom setting. |
Journal of Child & Adolescent Psychopharmacology 2005; 15(4):637-654. |
|
Supplementary randomised trials: Ritalin LA and MPH-IR |
||
|
Favreau et al, 2006 |
[Benefit of the extended-release methylphenidate formulations: a comparative study in childhood]. [French]. Plus English translation |
Archives de Pediatrie 2006; 13(5):442-448. |
8. Results of Trials
The results of the key trials are summarised in the tables below. The first table
summarises the changes from baseline at particular time points in the SKAMP (Swanson,
Kotkin, Agler, M-Flynn and Pelham) attention score (Swanson et al 1978; Swanson et
al 1998; Wigal et al 1978).
SKAMP-attention score: changes at different time-point test from pre-dose (efficacy
population)
|
Parameter |
Comparison |
Adjusted LS Means |
Difference of LS Means (SE) |
P-Value |
||
|---|---|---|---|---|---|---|
|
|
Ritalin LA |
Concerta |
Ritalin LA (SE) |
Concerta (SE) |
|
|
|
Trial: US05: Ritalin (N=36), Concerta (N=36) |
||||||
|
2.0 hour, change b |
20mg |
18mg |
-0.870 (0.114) |
-0.375 (0.114) |
-0.495 (0.157) |
0.002 |
|
4.0 hour, changeb |
20mg |
18mg |
-0.551 (0.113) |
-0.310 (0.113) |
-0.241 (0.157) |
0.129 |
|
8.0 hour, change c |
20mg |
18mg |
-0.338 (0.124) |
-0.500 (0.124) |
0.162 (0.175) |
0.356 |
|
Trial: US07: Ritalin (N=54), Concerta (N=53) |
||||||
|
2.0 hour, changea |
20mg |
18mg |
-0.897 (0.124) |
-0.689 (0.125) |
-0.207 (0.167) |
0.215 |
|
4.0 hour, changeb |
20mg |
18mg |
-0.681 (0.117) |
-0.443 (0.118) |
-0.238 (0.160) |
0.138 |
|
8.0 hour, changec |
20mg |
18mg |
-0.220 (0.128) |
-0.368 (0.130) |
0.147 (0.168) |
0.380 |
|
12.0 hour, change c |
20mg |
18mg |
0.140 (0.137) |
-0.288 (0.139) |
0.428 (0.195) |
0.029 |
Note: a = primary outcome, b=secondary outcome, c= exploratory analysis.
The second table summarises the change in scores as reflected in the area under the
curve (AUC) for SKAMP attention scores over different time periods.
Table SKAMP-attention AUC change from pre-dose (efficacy population)
|
Parameter |
Comparison |
Adjusted LS Means |
Difference of LS Means |
P-Value |
|||
|---|---|---|---|---|---|---|---|
|
|
Ritalin LA |
Concerta |
Ritalin LA (SE) |
Concerta (SE) |
|
|
|
|
Trial: US05: Ritalin (N=36), Concerta (N=36) |
|||||||
|
AUC(0-4) |
20mg |
18mg |
-2.481 (0.334) |
-1.362 (0.334) |
-1.119 (0.454) |
0.015 |
|
|
AUC(0-8)changec |
20mg |
18mg |
-4.481 (0.707) |
-2.719 (0.707) |
-1.763 (0.976) |
0.074 |
|
|
Trial: US07: Ritalin (N=54), Concerta (N=53) |
|||||||
|
AUC(0-4) change b |
20mg |
18mg |
-2.516 (0.349) |
-1.672 (0.353) |
-0.844 (0.469) |
0.022 |
|
|
AUC(0-8) change c |
20mg |
18mg |
-4.434 (0.720) |
-3.405 (0.727) |
-1.029 (0.973) |
0.291 |
|
|
AUC(8-12) |
20mg |
18mg |
-0.396 (0.450) |
-1.592 (0.455) |
1.197 (0.640) |
0.063 |
|
|
AUC(0-12) |
20mg |
18mg |
-4.831 (1.088) |
-4.994 (1.099) |
0.163 (1.500) |
0.913 |
|
Note: A=Ritalin LA 20 mg capsule, B=Ritalin LA 40 mg capsule,C =Concerta 18 mg tablet,
D=Concerta 36 mg tablet, E=Placebo.
a = primary outcome, b=secondary outcome, c= exploratory analysis.
The improvements in SKAMP attention scores for patients taking Ritalin LA 40 mg, as
reflected in differences at the 2, 4, and 8 hour time points and in the AUC values
at 4, 8 and 12 hours after the test dose, were significantly greater than for those
taking Concerta 18 mg or Concerta 36 mg. However, the efficacy diminished at 12 hours
post dose (top table).
The results of comparisons with the Ritalin LA 20mg were less clear cut. Ritalin LA
20mg achieved statistically greater improvement from pre-dose than Concerta 18mg and
36mg at hours 2 and 4 and AUC (0-4). However, when analysing the data in the 8 to
12 hour range post dose, Ritalin 20 was generally outperformed by Concerta 18mg. The
change scores at specific time points and the AUC values from 8 to 12 hours after
dosing (AUC (8-12)) show this trend more clearly. After 8 hours (the 8 and 12 hour
post dose time points), all efficacy outcomes tended to be greater with Concerta 18mg
and 36mg compared with Ritalin LA 20mg strength (top table).
The differences between the drugs in the time course of improvements in SKAMP-Attention
scores in trials US05 and US07 can be seen graphically in the figures presented below:
Average SKAMP –attention score over time: change from pre-dose (US05)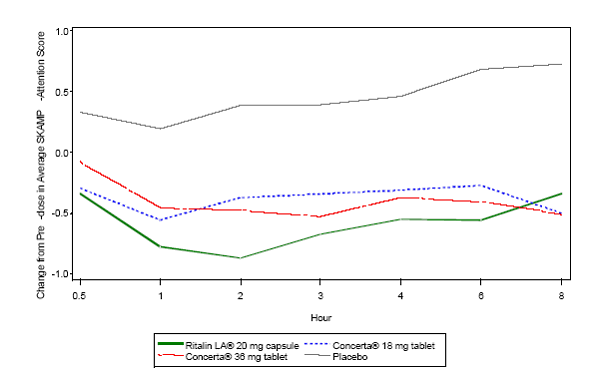
Average SKAMP attention score over time of US07: Change from pre-dose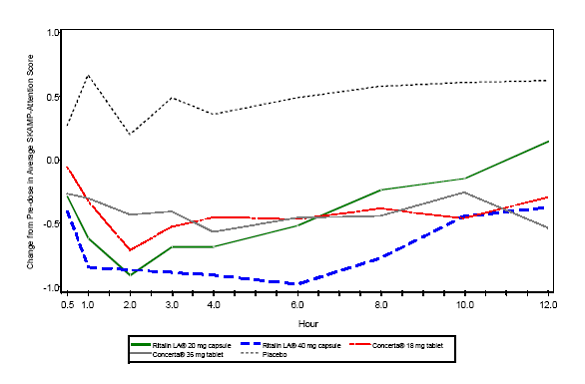
All treatment groups were statistically superior to placebo.
For PBAC’s comments on these results, see Recommendation and Reasons.
Comparative toxicity
All events were rated as mild to moderate in severity and occurred in different patients
in the randomised cross-over studies of Ritalin LA and Concerta.
The two supplementary studies showed that there were no major differences in safety
between Ritalin LA and MPH-IR.
9. Clinical Claim
The submission described Ritalin LA as being no worse than Concerta in terms of effectiveness and toxicity. The PBAC however concluded that the submission’s claim of non-inferiority had not been established, see Recommendation and Reasons.
10. Economic Analysis
Two different methods for determining the equi-effective doses of Ritalin LA and Concerta
were presented.
Although not directly relevant to its decision, the PBAC noted that the limited clinical
evidence provided make it difficult to determine the equi-effective doses of Ritalin
LA and Concerta.
11. Estimated PBS Usage and Financial Implications
The financial cost/year to the PBS (excluding co-payments) minus any savings in use of other drugs was estimated by the submission to be < $10 million (dangerous drug fee included) in each of the first four years.
12. Recommendation and Reasons
The PBAC noted that Ritalin LA controlled release methylphenidate has a different
pharmacokinetic profile to Concerta controlled release methylphenidate, against which
it is compared in this submission, and that the submission bases its claim that Ritalin
LA is no worse than Concerta on the results of two randomised cross-over trials in
which a total of 90 children received single doses of different strengths of the two
products. The Committee considered that the design of these clinical trials does not
allow extrapolation of the evidence into real life clinical practice where patients
will be treated on an ongoing basis, and thus that the submission’s claim of non-inferiority
had not been established.
The Committee also considered that immediate release methylphenidate is an appropriate
additional comparator and that a clinical comparison of Ritalin LA against twice daily
immediate release methylphenidate would have been informative. The PBAC recalled that
when recommending Concerta for listing, the Committee had agreed that the likely improvements
in compliance and ease of administration, particularly in relation to the removal
of the need for a dose of medication at school, were sufficient to justify listing
even though the extent of any clinical benefit of Concerta over immediate release
methylphenidate remained uncertain.
The PBAC rejected the submission because it did not include sufficient evidence to
enable it to conclude that Ritalin LA is non-inferior to Concerta, nor to determine
the effectiveness of Ritalin LA compared with immediate release methylphenidate.
Recommendation
Reject
13. Context for Decision
The PBAC helps decide whether and, if so, how medicines should be subsidised in Australia. It considers submissions in this context. A PBAC decision not to recommend listing or not to recommend changing a listing does not represent a final PBAC view about the merits of the medicine. A company can resubmit to the PBAC or seek independent review of the PBAC decision.
14. Sponsor’s Comment
The sponsor has no comment.




