Natalizumab, concentrated solution for IV infusion, 300 mg per 15 mL, Tysabri, November 2007
Public summary document for Natalizumab, concentrated solution for IV infusion, 300 mg per 15 mL, Tysabri, November 2007
Page last updated: 29 February 2008
Public Summary Document
Product: Natalizumab, concentrated solution for IV infusion, 300 mg per 15 mL, Tysabri
Sponsor: Biogen Idec Australia Pty Ltd
Date of PBAC Consideration: November 2007
1. Purpose of Application
The submission sought a Section 100 (Highly Specialised Drug) listing for initial
and continuing treatment, by neurologists, of clinically definite relapsing-remitting
multiple sclerosis (RRMS) in ambulatory patients eighteen years of age or older.
Highly Specialised Drugs are medicines for the treatment of chronic conditions, which,
because of their clinical use or other special features, are restricted to supply
to public and private hospitals having access to appropriate specialist facilities.
2. Background
At the November 2006 PBAC meeting, the PBAC rejected a submission seeking section 100 listing of natalizumab for initial and continuing treatment, by neurologists, of clinically definite relapsing-remitting multiple sclerosis (RRMS) in ambulatory patients eighteen years of age or older because of high and uncertain estimated base case cost-effectiveness ratio.
3. Registration Status
Natalizumab was TGA registered on 1 November 2006 as monotherapy for the treatment of patients with relapsing-remitting multiple sclerosis to delay the progression of physical disability and to reduce the frequency of relapse. The safety and efficacy of natalizumab beyond two years is unknown.
4. Listing Requested and PBAC’s View
Section 100
Highly Specialised Drugs
Public and Private Hospital Authority Required
Caution:
Progressive multifocal leukoencephalopathy (PML) has been reported with this drug.
Initial treatment, by neurologists, of clinically definite relapsing-remitting multiple
sclerosis in ambulatory (without assistance or support) patients eighteen years of
age or older, who have experienced at least 2 documented attacks of neurological dysfunction,
believed to be due to multiple sclerosis, in the preceding 2 years.
The diagnosis must be confirmed by magnetic resonance imaging of the brain and/or
spinal cord and the date of the scan included in the authority application, unless
the authority application is accompanied by written certification provided by a radiologist
that an MRI scan is contraindicated because of the risk of physical (not psychological)
injury to the patient.
A maximum quantity of 6 infusions will be issued with the initial authority (1 + 5
repeats).
Continuing treatment of clinically definite relapsing-remitting multiple sclerosis
in patients previously issued with an authority prescription for this drug who do
not show continuing progression of disability while on treatment with this drug, and
who have demonstrated compliance with, and an ability to tolerate, this therapy.
A maximum quantity of 3 infusions will be issued with the continuing authority (1
+ 2 repeats).
For PBAC’s view of the requested restriction, see Recommendation and Reasons.
5. Clinical Place for the Proposed Therapy
Multiple sclerosis (MS) is an incurable, degenerative disease of the brain and spinal
cord, characterised by loss of areas of nerve-fibre covering (demyelination) and nerve
(axonal) destruction.
Advancement in the treatment of multiple sclerosis occurred in the early and mid 1990’s
following the introduction of immunotherapy in the form of recombinant beta-interferons
and, later, glatiramer acetate. Prior to this, the only mainstream therapies available
for multiple sclerosis were oral or intravenous corticosteroids for the management
of acute relapses, and a range of antispasmodics, antidepressants and other therapies
for symptom management.
Natalizumab is proposed as a further disease-modifying immunotherapy agent, specifically
directed at an early step in the formation of the inflammatory lesion: migration of
white cells across the blood brain barrier.
6. Comparator
The submission nominated interferon beta-1b as the main comparator. This was previously accepted by PBAC. A comparison with interferon beta-1a was conducted during the evaluation.
7. Clinical Trials
No changes had been made to the trial data presented in the previous submission.
The key trials published at time of submission were as follows:
|
Trial/First author |
Protocol title |
Publication citation |
|---|---|---|
|
C-1801 AFFIRM |
A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. |
New England Journal of Medicine, 2006; 354: 899-910 |
|
INFN beta-1b |
||
|
IFNB MSSG trial |
Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. |
Neurology, 1993; 43(4 I): 655-661 |
|
Duquette P et al (1995) |
Interferon beta-1b in the treatment of multiple sclerosis: Final outcome of the randomized controlled trial. |
Neurology, 1995; 45(7): 1277-1285 |
|
INFN beta-1a |
||
|
NS 26321 |
A phase III trial of intramuscular recombinant INFN beta as treatment for exacerbating-remitting multiple sclerosis: design and conduct of study and baseline characteristics of patients. |
Multiple Sclerosis Collaborative Research Group (MSCRG). Mult Scler 1995; 1(2):118-135. |
|
Intramuscular INFN beta-1a for disease progression in relapsing multiple sclerosis. |
Ann Neurol 1996; 39(3):285-294. |
|
|
Impact of INFN beta-1a on neurologic disability in relapsing multiple sclerosis. |
Neurology 1997; 49(2):358-363. |
|
|
INFN beta-1a |
||
|
PRISMS |
Randomised double-blind placebo controlled study of INFN (beta)-1a in relapsing/remitting multiple sclerosis. |
Lancet 1998; 352(9139):1498-1504. |
|
Glatiramer acetate |
||
|
Johnson 1995 |
Copolymer 1 reduces relapse rate and improves disability in relapsing- remitting multiple sclerosis: Results of a phase III multicenter, double- blind, placebo-controlled trial. |
Neurology 1995; 45(7):1268- 1276. |
|
Management of relapsing/remitting multiple sclerosis with copolymer 1 (Copaxone). |
Mult Scler 1996; 1(6):325-326. |
|
|
Extended use of glatiramer acetate (Copaxone) is well tolerated and maintains its clinical effect on multiple sclerosis relapse rate and degree of disability. |
Neurology 1998; 50(3):701-708. |
|
8. Results of Trials
Indirect comparison of natalizumab versus INFN beta-1b:
The PBAC had previously stated that there is uncertainty in relation to the comparability
of patients enrolled in the AFFIRM and the IFNB MSSG trials due to patients in the
former having been enrolled on the basis of the more recently developed McDonald criteria
as opposed to those enrolled in the older betaferon trial according to the Poser criteria
and also due to differences in the rates for annualised relapse and disease progression
in patients randomised to the placebo arms of the trials. The submission provided
data that demonstrated that 96% of patients enrolled in the AFFIRM trial according
to the McDonald criteria would have also satisfied the Poser criteria.
The indirect comparison of natalizumab versus INFN beta-1b for the outcome of annualised
relapse rate is presented below.
Results of the indirect comparison– annualised relapse rate
|
Trial |
Rate ratio |
Natalizumab |
Placebo |
INFN beta-1b |
Rate ratio |
Indirect estimate of effect |
|---|---|---|---|---|---|---|
|
Results at 1 year |
||||||
|
AFFIRM |
0.341 a |
N=627 |
N= 315 |
0.51 |
||
|
IFNB MSSG |
N=123 |
N=124 |
0.67 c (0.524-0.849) |
|||
|
Results at 2 years |
||||||
|
AFFIRM 2 years |
|
N=627 |
N= 315 |
|||
|
IFNB MSSG e |
N=110 |
N=107 |
0.720 c d |
0.444 |
||
|
N=112 |
N=115 |
0.66 |
0.485 |
|||
Bolded font indicates statistically significant differences
a From Poisson regression, adjusted for the number of relapses in the one year prior
to study entry, baseline EDSS (EDSS ≤3.5 versus >3.5), presence of Gd lesions and
age (<40 versus ≥40). Trial reports both adjusted and unadjusted rate ratios but 95%
CI only for adjusted data.
b Rates were annualised for actual time on study
c Calculated post-hoc by the submission.
d Reported in the 1995 published report of IFNB MSSG trial as a 28% decrease INFN
beta-1b vs placebo
e There are inconsistencies in results reported at 2 years for the INFB MSSG trial
data across the 1993 and 1995 published reports of this trial. Both sets of results
are presented.
The results of the indirect comparison of natalizumab and INFN beta-1b demonstrated
that patients treated with natalizumab have statistically significantly reduced relapse
rates at both 1 and 2 years compared with those treated with INFN beta-1b.
Indirect comparison of natalizumab versus all other PBS listed MS therapies:
To address this issue, the submission presented a meta-analysis of placebo controlled
trials for other PBS listed agents used in the treatment of RRMS. These results were
used to inform the effect of INFN beta-1b at 2 years on disease progression (defined
as progression sustained for 12 or 24 weeks, as determined by an increase in EDSS
score of ≥1 (or ≥1.5 if the baseline EDSS was zero in the AFFIRM trial)), in the modelled
economic evaluation.
A summary of the results of the indirect comparison of natalizumab versus all other
PBS listed MS therapies are presented below.
Summary of results of meta-analysis and indirect comparison with natalizumab
|
Trial ID |
Trials of natalizumab |
Trials of comparators |
Indirect |
||||
|---|---|---|---|---|---|---|---|
|
Treatment |
Nataliz |
Placebo |
Placebo |
Compara-tor |
Treatment |
||
|
Progression sustained for 12 weeks: natalizumab vs all available comparator data |
|||||||
|
AFFIRM, |
0.580 |
104/627 |
84/315 |
- |
- |
- |
- |
|
IFNB MSSG, |
- |
- |
- |
nr/123 |
nr/124 |
0.714 |
- |
|
AFFIRM, |
0.551 |
112/627 |
95/315 |
- |
- |
- |
- |
|
PRISMS, |
- |
- |
- |
nr/187 |
nr/184 |
0.696 |
- |
|
Johnson 1995 |
- |
- |
- |
31/126 |
27/125 |
0.878 |
- |
|
Pooled |
0.565 |
- |
- |
- |
- |
0.739 |
0.764 |
|
Progression sustained for 24 weeks: natalizumab vs all available comparator data |
|||||||
|
AFFIRM, |
0.457 |
69/627 |
69/315 |
- |
- |
- |
- |
|
NS26321-01, |
- |
- |
- |
36/143 |
24/158 |
0.628 |
|
|
PRISMS, |
- |
- |
- |
nr/187 |
nr/184 |
0.757 |
- |
|
Pooled |
0.457 |
- |
- |
- |
- |
0.686 |
0.666 |
Abbreviations: Nataliz, natalizumab; CI, confidence interval; HR, hazard ratio; nr,
not reported; PCDR, progression confirmed during a relapse; inc, included; exc, excluded
a natalizumab vs placebo
b Comparator (INFN-1b or INFN-1a (Avonex or Rebif) or glatiramer acetate) vs placebo
c natalizumab vs comparator
d Indirect comparison using the Bucher et al (1997) method
Summary of meta-analysed disability progression data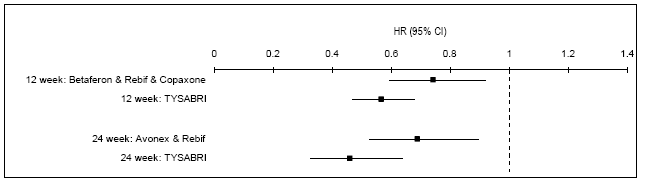
The results of the indirect comparison of natalizumab and all other PBS-listed MS
therapies indicated that the hazard ratio approached statistical significance (P=0.066)
for patients treated with natalizumab or all other PBS-listed MS therapies for disease
progression at 2 years when disease progression is sustained for 12 or 24 weeks.
During the evaluation process, the sponsor provided a re-analyses of the Rice et al
(2001) Cochrane review using an ITT approach for both study arms and used the revised
pooled estimate for progression sustained as the comparator in the indirect comparison
with natalizumab. The relative risk became 0.71 [95% CI: 0.54, 0.94] as opposed to
0.764 [95%CI: 0.574, 1.018]. This suggested that statistically significantly fewer
patients treated with natalizumab had progressed at two years compared with patients
treated with other MS drugs.
As the PBAC had indicated that a comparison of natalizumab versus INFN beta-1a would
be informative (see November 2006 Public Summary Document), this was conducted during
the evaluation. The results of the indirect comparison of natalizumab and INFN beta-1a
for annualised relapse rates demonstrated that patients treated with natalizumb had
statistically significantly reduced relapse rates at 2 years compared with those treated
with INFN beta-1a. There was no significant difference in the hazard ratio for patients
treated with natalizumab or INFN beta-1a for disease progression at 2 years when disease
progression is sustained for 12 or 24 weeks.
Overall, the PBAC noted that the results of the indirect comparisons indicate that
natalizumab has significant advantages over INFN beta-1b and INFN beta-1a (Avonex)
for a reduction in the relapse rate. No significant difference in disease progression
at 2 years (defined as disease progression is sustained for 12 or 24 weeks, as determined
by an increase in EDSS score of ≥1 (or ≥1.5 if the baseline EDSS was zero in the AFFIRM
trial) was demonstrated versus any comparator (INFN beta-1b, INFN beta-1a (Avonex)
and “all PBS-listed MS therapies”). However, these analyses were all based on indirect
comparisons and the trials were powered for differences in relapse rates rather than
progression of disease.
The submission presented an extended assessment of comparative harms with data from
more than 17,000 treated patients. The adverse events presented were generally consistent
with those reported in the AFFIRM trial.
9. Clinical Claim
The submission claimed that on the basis of the evidence presented, natalizumab monotherapy
is superior to interferon beta-1b in terms of efficacy and has similar or less toxicity.
The submission further implied that natalizumab had significant advantages over interferon
beta-1a and glatiramer acetate. The sponsor noted that two cases of progressive multifocal
leukoencephalopathy (PML) had occurred in other MS trials using natalizumab combination
therapy. No cases of PML had occurred in MS trials of natalizumab monotherapy.
For the PBAC’s view of this claim, see Recommendations and Reasons.
10. Economic Analysis
The submission calculated a trial-based incremental cost/additional progression-free
patient at 2 years of greater than $200,000 using surrogate estimates of effectiveness
for INFN beta-1b on disease progression.
An updated modelled economic evaluation was presented. The main difference from the
previous submission was that the effectiveness of INFN beta-1b was based on the meta-analysis
of all PBS listed therapies for MS.
The submission calculated a base case modelled incremental discounted cost/extra discounted
QALY over 64 years in the range $45,000 - $75,000.
11. Estimated PBS Usage and Financial Implications
The submission estimated that the number of patients treated would be less than 10,000
in Year 4 of listing.
The financial cost to the PBS was estimated to be in the range $30 - $60 million in
Year 4 of listing.
12. Recommendation and Reasons
The PBAC recommended the listing of natalizumab on the PBS for initial and continuing
treatment, by neurologists, of clinically definite relapsing-remitting multiple sclerosis
(RRMS) in an ambulatory patient eighteen years of age or older on the basis of a high
but acceptable cost-effectiveness ratio compared with interferon beta-1b.
The PBAC noted that the requested restriction is virtually the same as the current
restrictions for interferon beta-1b and interferon beta-1a. However, the PBAC considered
that the restriction for natalizumab should specify that it be used as monotherapy
as progressive multifocal leukoencephalopathy had not been reported in monotherapy
but was reported in combination therapy.
The PBAC agreed there was a high clinical need for natalizumab and that it offered
significant advantages over current therapies (interferon beta-1b and interferon beta-1a)
in terms of relapse rates, when the uncertainty arising from indirect comparisons
and the differences between the trial populations and trial design was taken into
account.
The PBAC also noted that the sponsor had proposed a risk sharing arrangement.
Recommendation
Natalizumab, concentrated solution for IV infusion, 300 mg per 15 mL
Section 100 Highly Specialised Drugs Program
Restriction: Private Hospital Authority Required
Caution:
Progressive multifocal leukoencephalopathy (PML) has been reported with this drug.
Initial treatment, as monotherapy, by neurologists, of clinically definite relapsing-remitting
multiple sclerosis in an ambulatory (without assistance or support) patient eighteen
years of age or older, who have experienced at least 2 documented attacks of neurological
dysfunction, believed to be due to multiple sclerosis, in the preceding 2 years.
The diagnosis must be confirmed by magnetic resonance imaging of the brain and/or
spinal cord and the date of the scan included in the authority application, unless
the authority application is accompanied by written certification provided by a radiologist
that an MRI scan is contraindicated because of the risk of physical (not psychological)
injury to the patient.
A maximum quantity of 6 infusions will be issued with the initial authority
Pack size:1
Continuing treatment, as monotherapy, of clinically definite relapsing-remitting multiple
sclerosis in a patient previously issued with an authority prescription for this drug
who do not show continuing progression of disability while on treatment with this
drug, and who have demonstrated compliance with, and an ability to tolerate, this
therapy.
A maximum quantity of 3 infusions will be issued with the continuing authority
Pack size: 1
13. Context for Decision
The PBAC helps decide whether and, if so, how medicines should be subsidised in Australia.
It considers submissions in this context. A PBAC decision not to recommend listing
or not to recommend changing a listing does not represent a final PBAC view about
the merits of the medicine. A company can resubmit to the PBAC or seek independent
review of the PBAC decision.
14. Sponsor’s Comment
Biogen Idec Australia welcomes the PBAC recommendation to list natalizumab (Tysabri) on the PBS.




