Sitagliptin phosphate, tablets, 100 mg, 50 mg and 25 mg, Januvia, March 2008
Sitagliptin phosphate, tablets, 100 mg, 50 mg and 25 mg, Januvia, March 2008
Page last updated: 04 July 2008
Public Summary Documents
Product: Sitagliptin phosphate, tablets, 100 mg, 50 mg and 25 mg, Januvia
Sponsor: Merck Sharp & Dohme (Australia) Pty Limited.
Date of PBAC Consideration: March 2008
1. Purpose of Application
The submission sought an Authority Required (Streamlined) listing for the dual combination treatment of type 2 diabetes mellitus:
- In combination with metformin when a combination of metformin and a sulfonylurea is contraindicated or not tolerated (Proposal A);
- In combination with a sulfonylurea where a combination of metformin and a sulfonylurea is contraindicated or not tolerated (Proposal B); and
- Both of the above and earlier in the treatment pathway where sitagliptin replaces a sulfonylurea in dual combination with metformin (Proposal C).
2. Background
This was the first time sitagliptin had been considered by the PBAC.
3. Registration Status
Sitagliptin was registered by the TGA on 14 January 2008 for the treatment of diabetes mellitus type 2 in persons 18 years of age and older who have failed dietary measures and exercise: as dual combination therapy with metformin, or with a sulfonylurea, or with a thiazolidinedione where the use of a thiazolidinedione is considered appropriate. .
4. Listing Requested and PBAC’s View
Proposal A
Authority Required (Streamlined)
Dual oral combination therapy with metformin:
Type 2 diabetes, in combination with metformin, in a patient whose HbA1c is greater
than 7% prior to initiation of sitagliptin despite treatment with metformin and where
a combination of metformin and a sulfonylurea is contraindicated or not tolerated.
Proposal B
Authority Required (Streamlined)
Dual oral combination therapy with a sulfonylurea:
Type 2 diabetes, in combination with a sulfonylurea, in a patient whose HbA1c is greater
than 7% prior to initiation of sitagliptin despite treatment with a sulfonylurea and
where a combination of metformin and a sulfonylurea is contraindicated or not tolerated.
Proposal C
Authority Required (Streamlined)
Dual oral combination therapy with metformin:
Type 2 diabetes, in combination with metformin in a patient whose HbA1c is greater
than 7% prior to initiation of sitagliptin despite treatment with metformin.
Dual oral combination with a sulfonylurea:
Type 2 diabetes, in combination with a sulfonylurea in a patient whose HbA1c is greater
than 7% prior to initiation of sitagliptin despite treatment with a sulfonylurea and
where a combination of metformin and a sulfonylurea is contraindicated or not tolerated.
The following text was suggested in the restriction for each of the proposals:
The date and level of the HbA1c must be documented in the patient's medical records
at the time sitagliptin treatment is initiated. The HbA1c must be no more than 4 months
old at the time sitagliptin treatment is initiated.
Note:
Sitagliptin is not PBS-subsidised for use in combination with metformin and a sulfonylurea
(triple oral therapy) or as monotherapy.
Blood glucose monitoring may be used as an alternative assessment to HbA1c levels
in the following circumstances:
(a) clinical conditions with reduced red blood cell survival, including haemolytic
anaemias and haemoglobinopathies; and/or
(b) red cell transfusion within the previous 3 months.
A patient in these circumstances will be eligible for treatment where blood glucose
monitoring over a 2 week period shows blood glucose levels greater than 10 mmol per
L in more than 20% of tests. The results of this blood glucose monitoring, which must
be no more than 4 months old at the time of initiation of sitagliptin therapy, must
be documented in the patient's medical records.
See Recommendation and Reasons for PBAC’s view.
5. Clinical Place for the Proposed Therapy
Sitagliptin is an orally-active inhibitor of the dipeptidyl peptidase 4 (DPP-4) enzyme
for the treatment of type 2 diabetes. Sitagliptin differs in chemical structure and
pharmacological action from glucagon-like peptide-1 (GLP-1) analogues, including insulin,
sulfonylureas or meglitinides, biguanides, thiazolidinediones, alpha-glucosidase inhibitors,
and amylin analogues.
Sitagliptin is indicated for the treatment of type 2 diabetes mellitus in patients
18 years of age and older to improve glycaemic control in combination with metformin,
a sulfonylurea, or thiazolidinediones, when diet and exercise, plus the single agent
do not provide adequate glycaemic control.
6. Comparator
The submission nominated rosiglitazone (Proposals A and B) and sulfonylureas (the earlier position in Proposal C) as the comparators. This was appropriate, however, the PBAC noted that insulin would also be an appropriate comparator as it is a treatment option at each point where sitagliptin therapy might be considered.
7. Clinical Trials
For Proposal A, the submission presented:
- one head-to-head trial of sitagliptin 100 mg versus rosiglitazone 8 mg (P801);
- an indirect comparison of five randomised controlled trials using placebo as the
common comparator [one trial of sitagliptin versus placebo (P020), three trials of
rosiglitazone versus placebo (Gomez-Perez 2000, Fonseca 2000, Lowry 2000), one trial
of pioglitazone versus placebo (Einhorn 2000)];
- an indirect comparison of three RCTs using sulfonylurea as the common comparator
[one trial of sitagliptin versus glipizide (P024), one trial of pioglitazone versus
gliclazide (Matthews 2005) and one trial of pioglitazone versus glimepiride (Umpierrez
2006)].
In addition, there were seven monotherapy trials to establish the dose relativity
of sitagliptin and rosiglitazone (P014, P021, P023, P036, Phillips 2001, Hanefeld
2007, Lebovitz 2001).
The dose relativity of 25 mg and 50 mg sitagliptin in patients with moderate and severe
renal failure is explored in study P028.
For Proposal B the submission presented:
- one trial of sitagliptin 100mg versus placebo (P035).
Proposal C the submission presented:
- one head-to-head trial of sitagliptin 100mg versus glipizide (P024 – also used for
Proposal A).
Published trials presented in the submission are listed in the table below.
|
Trial ID/First Author |
Publication title |
Publication citation |
|---|---|---|
|
Proposal A |
||
|
Indirect trials, placebo comparator |
||
|
Gomez-Perez FJ (2002) |
Efficacy and safety of rosiglitazone plus metformin in Mexicans with type 2 diabetes. |
Diabetes/Metabolism Research Reviews. 2002;18(2):127-134 |
|
Fonseca V |
Effect of metformin and rosiglitazone combination therapy in patients with Type 2 diabetes mellitus. A randomised controlled trial. |
JAMA 2000; 283(13):1695-1702 |
|
Einhorn D |
Pioglitazone hydrochloride in combination with metformin in the treatment of type 2 diabetes mellitus: A randomized, placebo-controlled study. |
Clinical Therapeutics. 2000;22(12):1395-1409 |
|
Indirect trials, dual therapy with a sulfonylurea – sulfonylurea comparator |
||
|
Matthews DR |
Long-term therapy with addition of pioglitazone to metformin compared with the addition of gliclazide to metformin in patients with type 2 diabetes: A randomized, comparative study. |
Diabetes/Metabolism Research Reviews. 2005;21(2):167-174 |
|
Umpierrez G (2006) |
Glimepiride versus pioglitazone combination therapy in subjects with type 2 diabetes inadequately controlled on metformin monotherapy: Results of a randomized clinical trial |
Current Medical Research & Opinion. 2006;22(4):751-759 |
|
Additional monotherapy studies for dose relativity |
||
|
Phillips LS |
Once- and twice-daily dosing with rosiglitazone improves glycemic control in patients with type 2 diabetes. |
Diabetes Care. 2001;24(2):308-315 |
|
Hanefeld M (2007) |
A one-year study comparing the efficacy and safety of rosiglitazone and glibenclamide in the treatment of type 2 diabetes. |
Nutrition, Metabolism & Cardiovascular Diseases. 2007;17(1):13-23 |
|
Lebovitz HE (2001) |
Rosiglitazone monotherapy is effective in patients with type 2 diabetes. |
Journal of Clinical Endocrinology and Metabolism 2001;86(1):280-8. |
8. Results of Trials
PROPOSAL A - dual combination therapy with metformin when a sulfonylurea is contraindicated
or not tolerated.
The trial results are presented below.
Results of Least-squares (LS) mean change from baseline in HbA1c (direct and indirect
trials)
|
Trial |
Mean % change (SD) from baseline in HbA1c |
Mean difference |
||
|---|---|---|---|---|
|
Drug |
Drug |
Drug |
||
|
Direct randomised controlled trials |
||||
|
P801 |
Sitagliptin 100mg |
Placebo |
Rosiglitazone 8mg |
-0.51 (-0.70, -0.32) |
|
Indirect trials, placebo common comparator |
||||
|
P020 |
Sitagliptin 100mg |
Placebo |
-0.65 (-0.77, -0.53) |
|
|
Gomez-Perez |
Rosiglitazone 4mg |
Placebo |
Rosiglitazone 8mg |
- 1.0% p=0.0132 |
|
Fonseca |
Rosiglitazone 4mg |
Placebo |
Rosiglitazone 8mg |
- 1.0% p<0.001 |
|
Lowry |
Placebo |
Rosiglitazone 8mg |
- 0.8% |
|
|
Einhorn |
Pioglitazone 30mg |
Placebo |
-0.83% |
|
|
Indirect trials, sulfonylurea common comparator |
||||
|
P024 |
Sitagliptin 100mg |
Glipizide 5-20mg |
-0.01(-0.09, 0.08) |
|
|
Matthews |
Pioglitazone 15-45mg |
Gliclazide 80-320mg |
0.02 (-0.15, 0.19) |
|
|
Umpierrez |
Pioglitazone 30-45mg |
Glimepiride 2-8mg |
0.07 (-0.28, 0.13) |
|
Abbreviations: NR = Not reported.
The data suggested that metformin plus sitagliptin produced a statistically significant
larger reduction in HbA1c than metformin plus placebo (P801, P020); and comparable
reductions in HbA1c to rosiglitazone 8mg (P801) and glipizide 5-20mg (P024) when added
to metformin. The addition of pioglitazone to metformin therapy produced similar reductions
in HbA1c to the addition of gliclazide (Matthews, 2005) and glimepiride (Umpierrez,
2006). HbA1c reductions were significantly greater with rosiglitazone 8mg/day than
rosiglitazone 4mg/day (Gomez-Perez, Fonseca).
The proportion of patients achieving target HbA1c levels (<7%, <8%) were greater with
metformin plus sitagliptin than metformin plus placebo (P801, P020), and were comparable
for sitagliptin and sulfonylureas (rosiglitazone 8 mg P801; glipizide P024) added
to metformin. The proportion of patients achieving target HbA1c levels were similar
for pioglitazone and glimepiride (Umpierrez, 2006).
The results of studies P801 and P020 suggest quantitatively larger reductions in HbA1c
with higher baseline HbA1c levels.
Sitagliptin plus metformin produced statistically significant larger reduction in
fasting plasma glucose than metformin plus placebo (P801, P020) and comparable reductions
to glipizide plus metformin (P024). In the only trial directly comparing sitagliptin
and rosiglitazone (P801), rosiglitazone 8 mg plus metformin produced a statistically
significant greater reduction in fasting plasma glucose (FPG) than did sitagliptin
plus metformin.
Sitagliptin plus metformin increased low-density lipoprotein cholesterol (LDL-C) levels
more than glipizide plus metformin (10.4% vs. 7.3%, NS, study P024), but the combination
produced smaller increases in LDL-C than did rosiglitazone plus metformin (11.4% vs.
26.2%; study P801).
Sitagliptin plus metformin produced statistically significant larger increase in high-density
lipoprotein cholesterol (HDL-C) than did glipizide plus metformin (3.7% vs. 1.2%:
study P024), but the combination produced smaller increases in HDL-C than did rosiglitazone
plus metformin (4.3% vs. 9.2%; study P801).
The results for triglycerides were not consistent across the trials. In the head-to-head
trial of sitagliptin plus metformin vs. rosiglitazone 8 mg plus metformin, sitagliptin
produced a statistically significant greater reduction in triglycerides (-4.8% vs.
13.1%, study P801).
Sitagliptin reported rates of hypoglycaemia were very low and comparable to placebo
and rosiglitazone treated patients.
One percent of sitagliptin treated patients had a body weight increase >3kg over 18
weeks compared to 2.4% of the placebo and 20.9% of the rosiglitazone treated group
(P801). Rosiglitazone treated patients had similar numerical weight increases to pioglitazone
and sulfonylurea treated patients (study P801, Fonseca, P024, Matthews, Umpierrez).
There was only limited data to examine the effects of sitagliptin on systolic blood
pressure.
PROPOSAL B: dual combination therapy with a sulfonylurea when metformin is contraindicated or
not tolerated.
The trial results are presented below.
Results of Least-squares (LS) mean change from baseline in HbA1c at 24 weeks in subset
of patients on glimepiride
|
Mean % change (95% CI) from baseline in HbA1c |
Mean difference |
||
|---|---|---|---|
|
Drug |
Drug |
||
|
Study P035, all treated patients |
|||
|
Sitagliptin N = 102 |
Sitagliptin 100mg |
Placebo |
-0.57 (-0.82, -0.32) |
|
Study P035, stratified by baseline HbA1c |
|||
|
HbA1c <8% |
Sitagliptin 100mg |
Placebo |
-0.68 (-1.12, -0.23) |
|
HbA1c ≥8%<9% |
Sitagliptin 100mg |
Placebo |
-0.45 (-0.84, -0.06) |
|
HbA1c ≥9% |
Sitagliptin 100mg |
Placebo |
-0.62 (-1.13, -0.12) |
Sitagliptin plus glimepiride produced a statistically significant greater reduction
in HbA1c than placebo plus glimepiride. Reductions in HbA1c were numerically greater
with higher baseline HbA1c levels.
With respect to proportions of patients reaching HbA1c targets, while numerically
larger (sitagliptin 10.8%, placebo 8.7%), there were no statistically significant
differences in proportions achieving target HbA1c (<7%) at 24 weeks between the two
groups.
Sitagliptin produced a statistically significant larger reduction in fasting plasma
glucose than did placebo treatment in Study P035, but no statistically significant
differences in total cholesterol or HDL-cholesterol were reported.
The submission presented a meta-analysis of placebo controlled trials of sitagliptin
versus placebo when added to either sulfonylurea or metformin therapy. The pooled
reduction in HbA1c for sitagliptin plus metformin was -0.61% (-0.73, -0.48) compared
to -0.57% (-0.59,
-0.55) for sitagliptin plus glimepiride.
Drug-related adverse experiences were higher in the sitagliptin than placebo arm of
study P035 (14.9% versus 6.8%), as were serious adverse experiences (5.4% versus 3.7%).
In study P035, sitagliptin was associated with a small but statistically significant
increase in weight compared to placebo treatment (1.1 kg at 24 weeks), no statistically
significant difference in effects on SBP and no episodes of hypoglycaemia requiring
medical assistance were reported.
PROPOSAL C: The submission identified Protocol 024 as relevant for this proposal. The study
compares the addition of sitagliptin 100 mg or glipizide 5-20 mg to stabilised metformin
therapy.
The trial results are presented below.
Results of Least-squares (LS) mean change from baseline in HbA1c at 52 weeks
|
Mean % change (95% CI) from baseline in HbA1c |
Mean difference |
||
|---|---|---|---|
|
Sitagliptin 100mg |
Glipizide 5-20mg |
||
|
Study P024, per protocol patients* |
|||
|
Sitagliptin N = 382 |
-0.67(-0.75, -0.59) |
-0.67 (-0.75, -0.59) |
-0.01 (-0.09, 0.08) |
|
Study P024, stratified by baseline HbA1c |
|||
|
HbA1c <7% |
-0.26 |
-0.14 |
-0.12# |
|
HbA1c ≥7<8% |
-0.53 |
-0.59 |
0.06 NS |
|
HbA1c ≥8%<9% |
-1.13 |
-1.11 |
-0.02 NS |
|
HbA1c ≥9% |
-1.68 |
-1.76 |
0.08 NS |
* non-inferiority study, reporting of per-protocol results is appropriate
# significance of this value was not reported
There were no statistically significant differences in reductions in HbA1c, proportions
of patients achieving target HbA1c levels at 24 weeks, or increases in total cholesterol
between sitagliptin or glipizide therapy added to stable metformin doses. There was
a statistically significant increase in HDL-C with sitagliptin.
With regard to comparative toxicity, hypoglycaemic episodes, weight gain and changes
in systolic blood pressure were reported, as estimates of changes in these measures
and were used in the economic model.
Drug related adverse experiences were higher with glipizide than sitagliptin, but
the rates of serious adverse experiences and serious drug-related adverse experiences
were similar in the two groups in Study P024. Discontinuations due to adverse experiences
were slightly higher in the glipizide arm, but there were no discontinuations due
to drug-related or serious adverse experiences.
Sitagliptin was associated with a reduction in weight at 24 weeks, compared to an
increase in glipizide treated patients. While glipizide was associated with more hypoglycaemic
episodes than sitagliptin, few of the episodes required either non-medical or medical
assistance to manage them. The differences between sitagliptin and glipizide were
statistically significant across all three categories of episodes of hypoglycaemia.
The PBAC considered the key issue with the safety data is that most of the evidence
is short term (maximum reporting in this submission of 52 weeks). DPP-IV inhibitors
do not act through specific targets on target tissues, so the full metabolic effects
of sitagliptin will not be identified in short term studies. DPP-IV is expressed in
many tissues besides the gut mucosa including T-cells, endothelium, liver, kidney
and lung. This lack of longer term safety data is also reflected in the ADEC Resolution
9109 stating that approval of sitagliptin should be subject to the agreement to undertake
a pharmacovigilance plan acceptable to the TGA, especially addressing GIT tumours
and late cancers; pancreatitis; GIT ischaemia; cardiovascular endpoint data; dental
and skeletal effects; and the potential effects of any non-specificity of DPP4 antagonism.
As of 27 October 2007, the sponsor has informed the TGA that it has developed a Risk
Management Plan to monitor the overall safety profile of sitagliptin.
The PBAC considered that with respect to the comparative safety of sitagliptin over
rosiglitazone, the lack of longer term safety data in the submission was reasonably
addressed by the sponsor by the provision of long term safety data (104 weeks from
P024) and additional post-marketing surveillance data that became available during
the evaluation process.
9. Clinical Claim
The clinical claims for the three proposals were:
Proposal A: Sitagliptin is equivalent in terms of comparative effectiveness (reductions in HbA1c)
and superior in terms of comparative safety (smaller increases in total cholesterol:HDL
ratio, less increase in weight, lower risk of oedema) over rosiglitazone.
Proposal B: Sitagliptin is superior in terms of comparative effectiveness and similar in terms
of comparative safety over placebo. The effect of sitagliptin when used in combination
with a sulfonylurea is similar to the effect of Sitagliptin when used in combination
with metformin. Evidence provided in support of Proposal A was relevant to Proposal
B therefore the clinical claim is the same.
Proposal C: Sitagliptin is equivalent in terms of comparative effectiveness (reduction in HbA1c
and treating to target) and superior in terms of comparative safety (less hypoglycaemia,
less increase in weight, more favourable effect on lipid profile, less increase in
systolic blood pressure) over glipizide.
See Recommendations and reasons for PBAC’s views.
10. Economic Analysis
The PBAC questioned the validity of the coefficient of durability (COD) and its relevance
as a driver in the economic evaluation model. The COD was based on the rate of increase
in HbA1c from weeks 24 to 52 (i.e. following the initial drop in HbA1c). The rate
of increase for those using sitagliptin was slightly less compared with the rate for
glipizide (see figure and table below).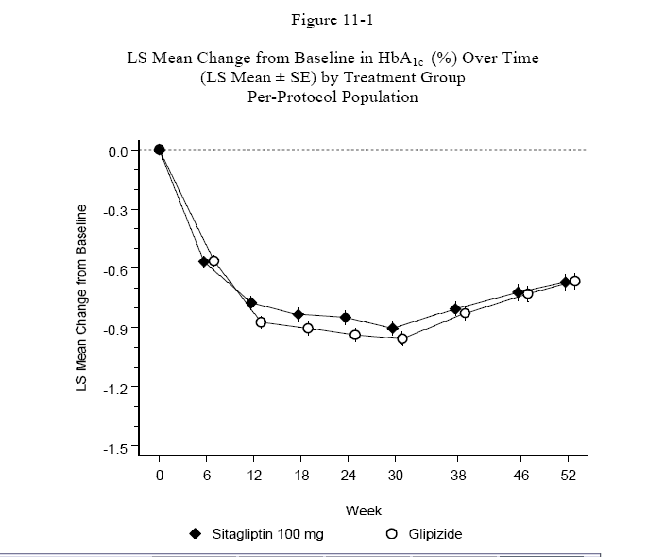
Coefficient of durability (using HbA1c LS means from week 24 to week 52)
|
Study P024 |
COD % per week (95% CI) |
Difference (95% CI) |
|
|---|---|---|---|
|
Sitagliptin 100mg |
Glipizide 5-20mg |
||
|
0.008 (0.005, 0.010) |
0.011 (0.008, 0.013) |
-0.003 (-.005, -0.001) p=0.005 |
|
The model assumes that the percent change per week in HbA1c continues over time (26
weeks נ0.008, i.e. increase in HbA1c of 0.208% for sitagliptin each 6 months and 26
x 0.011, increase in HbA1c of 0.286% for sulfonylurea). However, the PBAC considered
the validity of claims of persistence of effect on HbA1c over time was uncertain.
In relation to the COD in Study P024, the TGA Clinical Evaluator noted:
“In the report of Study P024V1, the author calculates “a coefficient of durability”
COD - essentially a measurement of the slope of the HbA1c time curve as it returns
towards baseline between 24 and 52 weeks. The author then argues for superiority of
sitagliptin over glipizide on the basis that the COD for the former is better (not
as steep). In my opinion, this procedure contributes nothing of value, if only because
the mean vertical coordinates of the relevant parts of the two curves do not coincide.
If such a claim of superiority is to be made, it must depend upon the acquisition
of further data.”
The 2-year update from trial P024 was provided by the sponsor. It suggested that the
COD has decreased in both arms of the trial, and stated that due to the trial design,
“over longer duration of P024 it will become increasingly difficult to demonstrate
any true differences in COD between the treatment groups.”
The submission presented a cost minimisation analysis to support Proposals A and B.
The equi-effective doses were estimated as sitagliptin 100 mg daily and rosiglitazone
8 mg daily. The conclusion was based on direct trial evidence and indirect evidence
using placebo controlled trials. The PBAC agreed that this was reasonable.
A modelled economic evaluation was presented in support of Proposal C. The model used
a Monte Carlo simulation of a Markov Model (TreeAge Pro Suite 2006). It used a lifetime
time horizon in the base case, with six month Markov cycles. Seven key clinical variables
drove the model – treatment discontinuations, HbA1c reductions, HbA1c coefficient
of durability (COD), hypoglycaemic episodes, changes in systolic blood pressure, TC:HDL
ratio, weight changes. The outcome derived is a cost/QALY.
The model was most sensitive to changes in the coefficient of durability (COD); removing
insulin costs; duration of model only 10 years; assumed changes in SBP; assuming no
difference in weight; and cost of sitagliptin. The incremental cost per QALY in the
base case was less than $15,000. The ICER increased to between $15,000 and $45,000
when the COD of sitagliptin is equal to that of a sulfonylurea (0.286) and to between
$45,000 and $75,000 when the COD of a sulfonylurea is set equal to that of sitagliptin
(0.208).
11. Estimated PBS Usage and Financial Implications
The estimated number of patients per year treated with sitagliptin was between 50,000 and 100,000 in Year 5. The net financial cost per year to the PBS was estimated in the submission to be less than $10 million in Year 5.
12. Recommendation and Reasons
Sponsor Proposals A and B
The PBAC recommended the listing of sitagliptin on the PBS for the treatment, as part
of dual oral combination therapy with metformin or a sulfonylurea, of a patient with
Type 2 diabetes whose HbA1c is greater than 7% prior to initiation of sitagliptin
despite treatment with metformin or a sulfonylurea and where a combination of metformin
and a sulfonylurea is contraindicated or not tolerated. Listing was recommended on
a cost-minimisation basis against rosiglitazone with the equi-effective doses being
sitagliptin 100 mg daily and rosiglitazone 8 mg daily.
In making this recommendation the PBAC noted that the data support the claim that
sitagliptin is no worse than rosiglitazone in terms of comparative effectiveness measured
as reductions in HbA1c. With respect to the comparative safety of sitagliptin over
rosiglitazone, the lack of longer term safety data in the submission was reasonably
addressed in the pre-PBAC response. The PBAC also noted that evidence provided in
support of Proposal A was relevant to Proposal B. Given the heterogeneity in the rosiglitazone
trials, it is reasonable to conclude that the incremental HbA1c reduction of adding
rosiglitazone to metformin is similar to that of adding rosiglitazone to a sulfonylurea.
Thus, there is a basis for comparing rosiglitazone with sitagliptin from the placebo-controlled
trial P035 presented as evidence for Proposal B. The PBAC agreed that the data available
suggest that sitagliptin is not associated with weight gain compared to increases
in weight of up to almost 2kg for rosiglitazone, pioglitazone and sulfonylureas. However,
there are no longer term data presented to confirm persistence of these effects on
body weight. The data on changes in systolic blood pressure are limited and it is
unclear that the reported changes represent clinically meaningful differences in blood
pressure response.
Sponsor Proposal C
The PBAC rejected the application to list sitagliptin on the PBS for use in Type 2
diabetes in combination with metformin where metformin treatment alone provides inadequate
control, and in the absence of a sulfonylurea contraindication or intolerance.
The Committee noted that acceptance of the claim that treatment with sitagliptin is
cost-effective compared with a sulfonylurea requires prior acceptance of the coefficient
of durability (COD) as a valid measure upon the basis of which the two treatments
can be extrapolated and clinically differentiated. The PBAC noted that the COD was
developed for use in a mathematical model only and there is currently no evidence
to demonstrate that the COD is a clinically relevant measure. The hearing confirmed
this view.
The PBAC also noted that, even if COD could be accepted as a valid measure, the 2-year
update from trial P024 provided by the Pre-Sub-Committee Response suggests that the
COD has decreased in both arms of the trial, and that due to the trial design, “over
longer durations of P024 it will become increasingly difficult to demonstrate any
true differences in COD between the treatment groups”. The model is extremely sensitive
to this variable. The ICER increases from less than $15,000 per QALY in the base case
to between $15,000 and $45,000 per QALY when the COD of sitagliptin is equal to that
of a sulfonylurea (0.286) or to between $45,000 and $75,000 per QALY when the COD
of a sulfonylurea is set equal to that of sitagliptin (0.208).
The PBAC also noted other concerns raised in respect of the economic model.
Thus overall, the PBAC rejected listing in the additional population covered by the
sponsors proposal C on the basis of uncertain evidence of a clinically relevant benefit
over the comparator, sulfonylureas, and because of the resulting highly uncertain
cost-effectiveness.
Recommendation
SITAGLIPTIN PHOSPHATE, tablets, 100 mg, 50 mg and 25 mg.
Restriction: NOTE:
Sitagliptin is not PBS-subsidised for use in combination with metformin and a sulfonylurea
(triple oral therapy), as monotherapy or in combination with a thiazolidinedione (glitazone).
Authority required
Dual oral combination therapy with metformin or a sulfonylurea.
Type 2 diabetes, in combination with metformin or a sulfonylurea, in a patient whose
HbA1c is greater than 7% prior to initiation of sitagliptin despite treatment with
either metformin or a sulfonylurea and where a combination of metformin and a sulfonylurea
is contraindicated or not tolerated.
The date and level of the HbA1c must be documented in the patient's medical records
at the time sitagliptin treatment is initiated. The HbA1c must be no more than 4 months
old at the time sitagliptin treatment is initiated.
Blood glucose monitoring may be used as an alternative assessment to HbA1c levels
in the following circumstances:
(a) clinical conditions with reduced red blood cell survival, including haemolytic
anaemias and haemoglobinopathies; and/or
(b) red cell transfusion within the previous 3 months.
A patient in these circumstances will be eligible for treatment where blood glucose
monitoring over a 2 week period shows blood glucose levels greater than 10 mmol per
L in more than 20% of tests. The results of this blood glucose monitoring, which must
be no more than 4 months old at the time of initiation of sitagliptin therapy, must
be documented in the patient's medical records.
Maximum quantity: 28
Repeats: 5
13. Context for Decision
The PBAC helps decide whether and, if so, how medicines should be subsidised in Australia. It considers submissions in this context. A PBAC decision not to recommend listing or not to recommend changing a listing does not represent a final PBAC view about the merits of the medicine. A company can resubmit to the PBAC or seek independent review of the PBAC decision.




