Sorafenib tosylate, tablet, 200 mg (base), Nexavar, March 2008
Public summary document for Sorafenib tosylate, tablet, 200 mg (base), Nexavar, March 2008
Page last updated: 04 July 2008
Public Summary Documents
Product: Sorafenib tosylate, tablet, 200 mg (base), Nexavar
Sponsor: Bayer Australia Ltd
Date of PBAC Consideration: March 2008
1. Purpose of Application
The submission sought an Authority Required listing for initial and continuing treatment of advanced renal cell carcinoma (RCC) in patients who meet certain criteria.
2. Background
At the November 2006 meeting, the PBAC rejected a submission for sorafenib based on uncertainty of the extent of gain in overall survival, and the resulting high and uncertain cost-effectiveness ratio. Overall, the PBAC accepted that there is a clinical need for additional treatment options for the management of renal cell carcinoma but that the data presented in the submission did not present, with an adequate degree of certainty, the extent of benefit that would be realised should sorafenib be listed on the PBS.
3. Registration Status
Sorafenib was TGA registered for the treatment of patients with advanced renal cell carcinoma on 27 September 2006.
4. Listing Requested and PBAC’s View
Authority Required
Initial treatment of advanced (unresectable or metastatic) renal cell carcinoma in
patients with WHO performance status 2 or less.
Continuing treatment of advanced renal cell carcinoma where the patient is not experiencing
(or is free of) disease progression.
Disease progression is defined as a 20% increase in the sum of the longest diameter
of target lesions using X-ray, CT or MRI.
Note: No applications for increased maximum quantities and/or repeats will be authorised.
See Recommendations and Reasons for PBAC’s view.
5. Clinical Place for the Proposed Therapy
Sorafenib is expected to be used alongside the current practice of best supportive care and in the minority of cases, it may replace immunotherapy or chemotherapy in the treatment of advanced renal cell carcinoma.
6. Comparator
The submission nominated placebo for best supportive care (BSC) as the main comparator.
Best supportive care consists of the use of pain medication, radiotherapy and other
supportive therapies. The PBAC had previously agreed that this is the appropriate
comparator.
The submission also nominated sunitinib as a secondary comparator. Although data from
trials located with a common comparator (interferon-alfa) were presented, no comparison
of the trials was provided, as it was not considered appropriate by the sponsor.
7. Clinical Trials
The re-submission presented an updated analysis of overall survival from Trial 11213.
Trial 11213 enrolled patients who had advanced RCC and had previously been treated
with one systemic therapy after which they experienced disease progression. Thus,
study 11213 provided evidence of the use of sorafenib as second line therapy where
other therapies have failed.
Two trials for the comparison of sorafenib versus sunitinib using interferon as a
common comparator were also presented, Trial 11848, comparing sorafenib and interferon-alfa,
and Motzer et al. 2007 comparing sunitinib and interferon alfa.
Trials published at the time of the re-submission are presented in the table below.
|
Trial/First author |
Protocol title/Publication title |
Publication citation |
|---|---|---|
|
Bukowski et al, 2007 |
Effects of sorafenib on symptoms and quality of life: Results from a large randomized placebo-controlled study in renal cancer. |
American Journal of Clinical Oncology: Cancer Clinical Trials 30(3): 220-227 |
|
Escudier et al, 2007 |
Sorafenib in advanced clear-cell renal-cell carcinoma. |
The New England Journal of Medicine 356(2): 125-134. |
|
Lamuraglia et al, 2006 |
To predict progression-free survival and overall survival in metastatic renal cancer treated with sorafenib: Pilot study using dynamic contrast-enhanced Doppler ultrasound. |
European Journal of Cancer 42(15): 2472-2479. |
|
Motzer et al, 2007 |
Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. |
The New England Journal of Medicine 356(2): 115-24. |
8. Results of Trials
The result of the overall survival analysis comparing sorafenib and BSC/placebo is
unchanged from the previous submission, i.e. no statistical significance was demonstrated.
However, ,progression-free survival reached significance. The results to 31 May 2005,
before cross-over, are the most relevant (Hazard Ratio (HR) = 0.71). The overall survival
(OS) results did not reach statistical significance as pre-specified by the trial
protocol.
The key results are summarised in the table below.
Overall survival results of Trial 11213
|
Level of statistical significance |
Other analyses |
||
|---|---|---|---|
|
Observed |
Needed* |
||
|
Current submission |
|||
|
Comparison of time-to-first event by 2-sided stratified log rank test |
P=0.146 |
Not provided but must be P<0.05 |
HR = 0.88 (0.74, 1.04) |
|
Same as above but with all placebo patients censored at 30 June 2006 |
Not included in the alpha spending plan |
HR=0.78 (0.62, 0.97) |
|
|
Previous submission |
|||
|
Comparison of time-to-event by 2-sided stratified log rank test |
P=0.018 |
P=0.0005 |
|
|
HR = 0.71 (0.54, 0.94)** |
|||
|
Previous submission |
|||
|
Comparison of time-to-event by 2-sided stratified log rank test |
P=0.015 |
P=0.0094 |
HR=0.77 (0.63, 0.95) |
Notes: HR=hazard ratio.
* Needed significance levels for interim analyses are dependent on the exact number
of events at the time of the analysis constrained by the overall apportionment of
the alpha which here was 0.01 for progression free survival (PFS) and 0.04 for overall
survival. The protocol and statistical analysis plan provided for one interim and
one final OS analysis plus one PFS analysis. After the PFS analysis of 28 January
2005, a decision was made in April 2005 to terminate randomized assignment and allow
crossover, and plan for three OS analyses: The first on 31 May 2005, a second on 30
November, and a final at 540 deaths which turned out to be on 8 September 2006
** The HR of 0.71 is reported in the current submission, whereas 0.72 was reported
in the previous submission. The discrepancy is explained by the current database being
more complete and up to date.
Trial 11848 was an open-label comparison of sorafenib and interferon-alfa in 189 patients
with previously untreated RCC. The primary outcome was progression-free survival by
independent review. Upon reaching progression, sorafenib patients underwent a dose
increase and interferon-alfa patients switched to sorafenib. There were 121 PFS events.
43 patients assigned to sorafenib had their dose increased and 50 patients assigned
to interferon-alfa were switched to sorafenib. The Kaplan-Meier curves are shown below
with a log-rank test of p=0.504. Median PFS was 5.9 months for sorafenib compared
to 5.6 months for interferon-alfa.
Trial 11848 Kaplan-Meier estimates for progression-free survival (by independent assessment)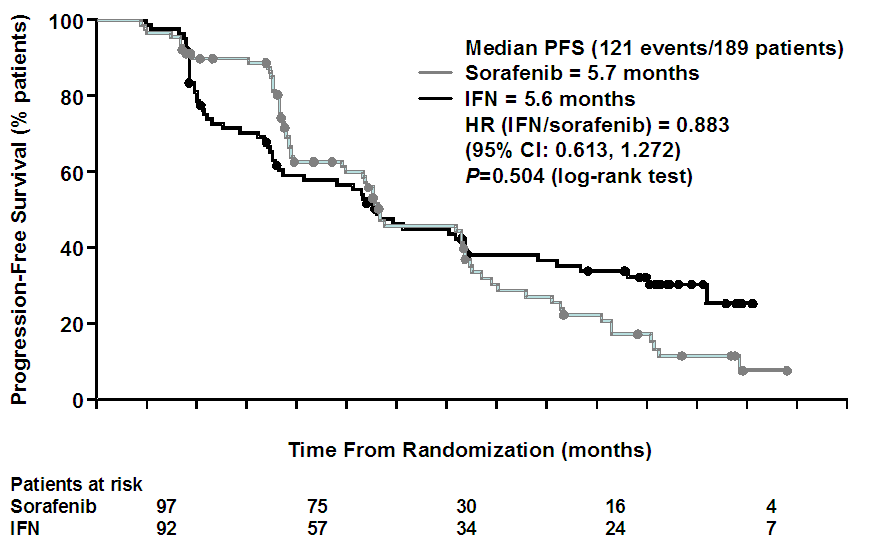
The above K-M curve suggested that sorafenib and interferon-alfa have similar progression
free survival. In addition, the PBAC noted a Cochrane review of interferon-alfa for
advanced RCC (Coppin et al. 2004) which concluded that interferon-alfa provided a
modest overall survival benefit compared to other commonly used treatments (overall
survival HR=0.74 (95% CI: 0.63 to 0.88); and weighted average median improvement in
survival was 3.8 months). The treatment effect for overall survival for interferon
vs. BSC as estimated in the Cochrane review was of similar magnitude to the treatment
effect for overall survival being claimed in this submission for sorafenib vs. BSC.
9. Clinical Claim
The re-submission described sorafenib as superior in terms of comparative effectiveness
and inferior in terms of comparative safety over BSC/placebo.
See Recommendations and Reasons for PBAC’s view.
10. Economic Analysis
A new modelled economic evaluation was presented which, compared with the model in
the previous submission, was 5 instead of 11 years in duration and modeled two health
states (alive and dead) instead of three (progression-free, disease progression and
dead).
The model used a more patient relevant outcome, survival, and thus avoided the problem
of limited evidence on the patient relevance of “progression”. However, the model
relied on an outcome, overall survival, the result of which failed to attain statistical
significance in the trial. Drivers of the model were the treatment effect and its
duration, the duration of sorafenib usage, and the utility assumed beyond the trial
duration.
The economic evaluation produced an incremental cost per extra QALY gained in the
range of $45,000 to $75,000.
11. Estimated PBS Usage and Financial Implications
The estimated likely number of patients per year was less than 10,000 in Year 1 at a financial cost per year to the PBS of between $10-$30 million in Year 1.
12. Recommendation and Reasons
The PBAC accepted that there is a clinical need for additional treatment options for
the management of renal cell carcinoma.
The requested listing for first line treatment was not supported by the pivotal trial
evidence presented in the submission, Trial 11213, which is in second line. Although
the re-submission implies that a first line listing is appropriate because the subgroup
analysis of patients without prior cytokine therapy showed improved survival compared
to those with such prior therapy, the subgroup analysis cannot add to this inference
because the overall survival analysis for the trial was not statistically significant,
the subgroup analysis was not pre-specified and the patient group was not adequately
defined. The only evidence supporting a treatment setting for sorafenib was in patients
in whom cytokine therapy had failed, although these therapies are not currently PBS
listed. Even in this setting, the evidence being relied on would be progression-free
survival, not overall survival.
The only first-line trial available (Trial 11848, which was open-label) showed no
benefit in progression-free survival or overall survival. The PBAC therefore considered
a PBS listing of sorafenib for first line use would not be appropriate.
The PBAC agreed that the data in Trial 11213 suggests that treatment with sorafenib
to improve progression free survival, however considered that the clinical importance
of this gain had not been demonstrated in the submission, either in terms of symptoms
of renal cell carcinoma or as a surrogate to predict future survival gain. Although
in clinical practice there may be a select population in whom there is a survival
benefit, the 4 month survival benefit claimed in the submission remained uncertain.
The Committee noted the influence that the cross over from placebo to sorafenib treatment
in the pivotal trial (Trial 11213), had on the ability of the submission to demonstrate
efficacy in terms of the extent of overall survival gain compared to placebo.
The PBAC noted that only 9.5% of patients achieved a partial response to sorafenib
as compared to 1.8% for placebo, in the comparison of RECIST categories determined
by the investigator. The clinical rationale for patients not achieving a partial response
was unknown.
The PBAC noted that sorafenib is associated with a variety of adverse events and laboratory
findings including dermatologic and gastrointestinal events, hypertension, sensory
neuropathy, and neutropenia. Additionally, a six-fold increase in cardiac ischaemia/infarction
was found in Trial 11213 for sorafenib treated patients compared to placebo. Diarrhoea,
rash, fatigue, hand-foot syndrome, alopecia and nausea were reported in >20% patients.
While the modelled economic evaluation was technically sound in terms of the decision
to use overall survival rather than progression-free survival, issues of uncertainty
remained from the extrapolation of the overall survival data from the clinical evidence.
In the previous submission considered in November 2006, the key concern raised by
the modelled economic evaluation related to the time horizon where nine to twelve
months’ worth of data were extrapolated to eleven years. The model structure in the
current re-submission had improved, with a shorter time horizon of 5 years and a price
reduction of DPMQ. However, these changes were not enough to offset the clinical uncertainties
and the ICERs remained unacceptably high.
The PBAC therefore rejected the submission based on an unacceptably high and uncertain
cost effectiveness ratio. There is high clinical uncertainty associated with the claimed
survival advantage, but the place of sorafenib in the treatment algorithm is uncertain.
13. Context for Decision
The PBAC helps decide whether and, if so, how medicines should be subsidised in Australia. It considers submissions in this context. A PBAC decision not to recommend listing or not to recommend changing a listing does not represent a final PBAC view about the merits of the medicine. A company can resubmit to the PBAC or seek independent review of the PBAC decision.




