Adalimumab, injection, 40 mg in 0.8 mL pre-filled syringe, 40 mg in 0.8 mL pre-filled pen, Humira®, July 2008
Public summary document for Adalimumab, injection, 40 mg in 0.8 mL pre-filled syringe, 40 mg in 0.8 mL pre-filled pen, Humira®, July 2008
Page last updated: 14 November 2008
Public Summary Documents
Product: Adalimumab, injection, 40 mg in 0.8 mL
pre-filled syringe, 40 mg in 0.8 mL pre-filled pen,
Humira®
Sponsor:Abbott Australasia Pty Ltd
Date of PBAC Consideration: July 2008
1. Purpose of Application
The application sought to extend the current section 85 Authority
required listing to include treatment of severe chronic plaque
psoriasis.
2. Background
Adalimumab is currently listed on the Pharmaceutical Benefits
Scheme (PBS) for the treatment of rheumatoid arthritis, psoriatic
arthritis and ankylosing spondylitis and had not previously been
considered for treatment of chronic plaque psoriasis.
Adalimumab was recommended for listing for treatment of Crohn
disease at the November 2007 Pharmaceutical Benefits Advisory
Committee (PBAC) meeting and the listing implemented from 1 August
2008.
3. Registration Status:
Adalimumab was registered 17 April 2008 for the treatment of
moderate to severe chronic plaque psoriasis in adult patients who
are candidates for systemic therapy or phototherapy.
Adalimumab is also TGA registered for:
- Rheumatoid Arthritis
- Psoriatic Arthritis
- Ankylosing Spondylitis
- Crohn Disease
4. Listing Requested and PBAC’s View
The requested listing was identical to that of the current PBS
listings for etanercept, efalizumab and infliximab for the
treatment of chronic plaque psoriasis.
5. Clinical place for the Proposed Therapy
Psoriasis is a chronic, relapsing inflammatory skin condition of
which the plaque type is the most common. Plaque psoriasis is
characterized by scaling and inflammation with circular-to-oval red
plaques distributed most commonly on the scalp, elbows, knees, and
lower back. The inflammation may also affect the fingernails,
toenails, soft tissues of the mouth, genitalia, and joints. The
inflamed areas cause pain, itching, and discomfort. The extent and
duration of the disease is highly variable and can be characterised
by acute flare ups and relapses.
Adalimumab is a biological Disease-modifying Antirheumatic Drug
(bDMARD). bDMARDS are used to slow down the progression of the
disease. Adalimumab will provide clinicians with an alternative
bDMARD therapy for patients suffering with severe plaque psoriasis
whose condition is refractory to other systemic treatments or
phototherapy.
For PBAC’s view, see Recommendation and
Reasons.
6. Comparator
The submission nominated infliximab as the main comparator and
efalizumab as a secondary comparator.
For PBAC’s view, see Recommendation and
Reasons.
7. Clinical Trials
The submission presented indirect comparisons using meta-analyses of three sets of
randomised trials for adalimumab (M02-528, M03-656 REVEAL and M04-716 CHAMPION), infliximab
(Chaudhari, Gottlieb, EXPRESS and EXPRESS II) and efalizumab (CLEAR, Gordon, Lebwohl,
Leonardi and Papp). Placebo was used as the common comparator.
The following table lists the randomised trials published at the time of the submission.
| Trial ID | Protocol title/ Publication title | Publication citation |
|---|---|---|
| ADALIMUMAB | ||
| M03-656 (REVEAL) Menter A et al | Adalimumab therapy for moderate to severe psoriasis: a randomized, controlled phase III trial. | Journal of American Academy of Dermatology, 2008; 58(1): 106–115. |
| Revicki D.A., et al | Impact of adalimumab treatment on patient-reported outcomes: results from a Phase III clinical trial in patients with moderate to severe plaque psoriasis. | Journal of Dermatological Treatment, 2007; 18: 341–350 |
| EFALUZIMAB | ||
| CLEAR Dubertret-L, et al, | Clinical experience acquired with the efalizumab (Raptiva) (CLEAR) trial in patients with moderate-to-severe plaque psoriasis: results from a phase III international randomized, placebo-controlled trial. | The British journal of dermatology, 2006; 155(1): 170-181 |
| Ortonne-Jean-Paul, et al. | Impact of efalizumab on patient-reported outcomes in high-need psoriasis patients: results of the international, randomized, placebo- controlled Phase III Clinical Experience Acquired with efalizumab (CLEAR) trial (NCT00256139). | BMC dermatology, 2005; 5: 13. |
| Sterry-Wolfram, et al | Clinical Experience Acquired with efalizumab (CLEAR) trial in patients with moderate-to-severe plaque psoriasis: results from extended treatment in an international, Phase III, placebo-controlled trial. | Journal der Deutschen Dermatologischen Gesellschaft - Journal of the German Society of Dermatology: JDDG, 2006; 4(11): 947-56. |
| Gordon-Kenneth-B., et al, 2003 | Efalizumab for patients with moderate to severe plaque psoriasis: a randomized controlled trial. | JAMA : the journal of the American Medical Association, 2003; 290(23): 3073-80 |
| Menter-Alan et al | Efficacy and safety observed during 24 weeks of efalizumab therapy in patients with moderate to severe plaque psoriasis. | Archives of dermatology, 2005; 141(1): 31-8. |
| Lebwohl-Mark, et al, 2003 | A novel targeted T-cell modulator, efalizumab, for plaque psoriasis. | The New England journal of medicine, 2003; 349(21): 2004-13. |
| Leonardi-Craig-L., et al, 2005 | Extended efalizumab therapy improves chronic plaque psoriasis: results from a randomized phase III trial. | Journal of the American Academy of Dermatology, 2005; 52(3 Pt 1): 425-33 |
| Papp-Kim-A et al, 2006 | Safety of efalizumab in adults with chronic moderate to severe plaque psoriasis: a phase IIIb, randomized, controlled trial. | International journal of dermatology, 2006; 45(5): 605-14 |
| INFLIXIMAB | ||
| Chaudhari-U et al, 2001 | Efficacy and safety of infliximab monotherapy for plaque-type psoriasis: a randomised trial. | Lancet; 2001; 357(9271): 1842-7 |
| Gottlieb-Alice-B et al | Infliximab monotherapy provides rapid and sustained benefit for plaque-type psoriasis. | Journal of the American Academy of Dermatology; 2003a; 48(6): 829-35 |
| Gottlieb-A-B et al | Pharmacodynamic and pharmacokinetic response to anti-tumor necrosis factor-(alpha) monoclonal antibody (Remicade) treatment of moderate to severe psoriasis vulgaris. | Journal of the American Academy of Dermatology; 2003b; 48(1): 68-75 |
| Gottlieb-Alice-B et al, 2004 | Infliximab induction therapy for patients with severe plaque-type psoriasis: a randomized, double-blind, placebo-controlled trial. | Journal of the American Academy of Dermatology; 2004; 51(4):534-42 |
| Feldman-S-R et al | Infliximab treatment results in significant improvement in the quality of life of patients with severe psoriasis: a double-blind placebo- controlled trial. | The British journal of dermatology; 2005; 152(5): 954-60 |
| EXPRESS Reich-Kristian et al | Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. | Lancet; 2005; 366(9494): 1367-74 |
| Reich-K et al | Improvement in quality of life with infliximab induction and maintenance therapy in patients with moderate-to-severe psoriasis: a randomized controlled trial. | The British journal of dermatology; 2006; 154(6): 1161-8 |
| Reich-Kristian et al | Infliximab treatment improves productivity among patients with moderate-to-severe psoriasis. | European journal of dermatology; 2007; 17(5): 381-386 |
| EXPRESS II Menter-Alan et al | A randomized comparison of continuous vs. intermittent infliximab maintenance regimens over 1 year in the treatment of moderate-to- severe plaque psoriasis. | Journal of the American Academy of Dermatology; 2007; 56(1): 31.e1-15 |
| Leonardi C et al | Infliximab for the treatment of moderate to severe psoriasis: response to re-treatment. | Abstract P2888. American Academy of Dermatology 64th Annual Meeting March 3-7, 2006, J Am Acad Dermatol; 54: AB220 |
8. Results of Trials
Adalimumab vs. infliximab – 12 weeks
The submission presented a pooled odds ratio indirect comparison suggesting adalimumab
was inferior to infliximab in achieving a PASI 75 at week 12. However, an indirect
analysis conducted during the evaluation of the pooled relative risk suggested adalimumab
was non-inferior to infliximab in achieving a
PASI 75 at week 12.
The results of the indirect comparison of adalimumab versus infliximab (PASI 75 at
week 12) using odds ratios and relative risks are summarised in the following table:
| Adalimumab vs. placebo Pooled treatment effect (95% CI) | Infliximab vs. placebo Pooled treatment effect (95% CI) | Indirect estimate of effect (95% CI) |
|---|---|---|
| OR 31.93 (18.79, 54.24) | OR 118.57 (60.39, 232.83) | 0.2692 (0.11, 0.63) |
| RR 9.64 (4.36, 21.28)* | RR 17.40 (6.41, 47.19)* | 0.5540 (0.15, 1.98)* |
* Analyses conducted during the evaluation
Adalimumab vs. efalizumab – 12 weeks
Similarly, the submission presented a pooled odds ratio indirect comparison suggesting
adalimumab was superior to efalizumab in achieving a PASI 75 at week 12. However,
an indirect analysis conducted during the evaluation using the pooled relative risks
indicated that adalimumab was not superior to efalizumab but rather adalimumab was
non-inferior to efalizumab in achieving a PASI 75 at week 12.
The results of the indirect comparison of adalimumab versus efalizumab (PASI 75 at
week 12) using odds ratios and relative risks are summarised in the following table:
| Adalimumab vs. placebo Pooled treatment effect (95% CI) | Efalizumab vs. placebo Pooled treatment effect (95% CI) | Indirect estimate of effect (95% CI) |
|---|---|---|
| OR 31.93 (18.79, 54.24) | OR 9.96 (6.60, 15.02) | 3.2068 (1.64, 6.27) |
| RR 9.64 (4.36, 21.28)* | RR 7.34 (5.23, 10.30)* | 1.31 (0.55, 3.11)* |
* Analyses conducted during the evaluation
The PBAC considered that the pooled odds ratio was a more relevant measure for the
indirect comparison in the submission.
When analyses are conducted using relative risks, there were no statistically significant
differences in effect size between adalimumab and either infliximab or efalizumab.
In each case the confidence interval around the indirect estimate of effect was quite
wide.
Adalimumab vs. infliximab – 52 and 50 weeks
The submission also presented an indirect comparison of two trials (REVEAL and EXPRESS)
using odds ratios, suggesting adalimumab was non-inferior to infliximab in achieving
a PASI 75 at 52 weeks. The conclusion was similar using an indirect comparison using
relative risks.
The results of the indirect comparison of adalimumab versus infliximab (PASI 75 at
week 52) are summarised in the following table:
| Adalimumab vs. placebo Treatment effect (95% CI) | Infliximab vs. placebo Treatment effect (95% CI) | Indirect estimate of effect (95% CI) |
|---|---|---|
| OR 16.45 (9.74, 27.78) | OR 25.50 (7.87, 82.67) | 0.6452 (0.18, 2.34) |
| RR 9.47 (5.98, 14.99)* | RR 13.05 (4.28, 39.78)* | 0.7257 (0.22, 2.42)* |
* Analyses conducted during the evaluation
The 52-week data presented in the submission were difficult to interpret because of
the complex cross-over design of these trials, in combination with the interruption
and re-randomisation of the treatment and placebo arms in each case.
Both adalimumab and infliximab treatment arms showed a decrease in response over time.
The limited data available suggested there may be a smaller loss of response over
time with adalimumab compared with infliximab. Given the complexity of the two study
designs, definitive conclusions are not possible.
A table summarising the results of the indirect comparison of adalimumab to infliximab
of patients maintaining a PASI 75 response at 52 and 50 weeks respectively – Odds
Ratios (OR) is shown below:
| Trial ID | Adalimumab | Infliximab | Indirect estimate of effect (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | ADA n/N (%) | Placebo n/N (%) | Placebo n/N (%) | INF n/N (%) | OR (95% CI) | ||
| REVEAL 52 weeks | 16.45 (9.74, 27.78) | 113/250 (45.2%) | 19/398 (4.8%) | - | - | - | 0.6452 (0.18, 2.34) p=0.505 |
| EXPRESS 50 weeks | - | - | - | 3/77 (3.9%) | 153/301 (50.8%) | 25.50 (7.87, 82.67) | |
| Treatment effect a | 16.45 (9.74, 27.78) | 25.50 (7.87, 82.67) | |||||
Abbreviations: ADA = Adalimumab; INF = Infliximab
a pooled using the random effects model
An indirect comparison of treatment effect suggested no statistically significant
difference between adalimumab and infliximab at 52 weeks. However, the confidence
interval around the indirect estimate of effect was wide.
The table below shows the proportion of subjects maintaining a PASI 75 response at
various time points in REVEAL:
| Period A | Proportion of adalimumab patients achieving a PASI 75 response n/N (%) | |
|---|---|---|
| Week 12 a Week 16 b | 551/814 (67.7) 578/814 (70.9) | |
| Period B c | Proportion of week 16 PASI 75 responders on adalimumab maintaining a PASI 75 response n/N (%) | |
| Week 24 Week 33 | 538/580 (92.8) 490/580 (84.5) | |
| Period C d | Proportion of week 33 PASI 75 responders maintaining a PASI 75 response | |
| Adalimumab n/N (%) | Placebo n/N (%) | |
| Week 36 Week 40 Week 44 Week 48 Week 52 | 234/250 (93.6) 215/250 (86.0) 214/250 (85.6) 208/250 (83.2) 198/250 (79.2) | 220/240 (91.7) 186/240 (77.5) 158/240 (65.8) 135/240 (56.3) 102/240 (42.5) |
NB: missing values imputed as non-responders. a, b, c, dSource: REVEAL.
A table of the proportion of infliximab patients who were PASI 75 responders at week
10 and who maintained a PASI 75 response at week 24 and week 50 is shown below:
| Trial duration | Proportion of infliximab patients achieving a PASI 75 response n/N (%) |
|---|---|
| Week 10 | 242/301 (80.4) |
| Proportion of week 10 responders on infliximab maintaining a PASI 75 response n/N (%) | |
| Week 24 Intention to treat Evaluable patients Week 50 Intention to treat Evaluable patients | 203/242 (83.9) 203/229 (88.6) 153/242 (63.2) 153/225 (68.0) |
Alternative analyses
The results using evaluable patient populations for both adalimumab and infliximab
showed that infliximab was superior at 52 weeks (OR = 0.39, CI 0.27, 0.56). The results
using the ITT population for both adalimumab and infliximab numerically favoured infliximab,
but were not statistically significant (OR = 0.80, CI 0.62, 1.05).
The results of the Odds Ratios for the various therapies versus placebo at 52 weeks
are summarised in the following figure.
The results of the Odds Ratio for adalimumab versus infliximab – 52 weeks, ITT (adalimumab
814 patients; infliximab 301 patients) is shown in the figure below:
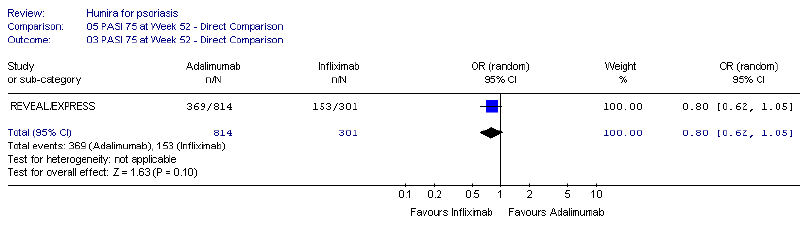
For PBAC’s comments on these results, see Recommendation and Reasons.
The use of pooled odds ratios in preference to pooled relative risks impacted significantly
on the results of the indirect comparison of adalimumab and infliximab.
A table summarising the results of the indirect comparison of adalimumab versus infliximab
adverse events – Odds Ratios (OR) is shown below:
| Adalimumab vs. placebo Pooled treatment effect (95% CI) | Infliximab vs. placebo Pooled treatment effect (95% CI) | Indirect estimate of effect (95% CI) |
|---|---|---|
| OR 1.09 (0.74, 1.59) | OR 1.82 (1.37, 2.42) | 0.5983 (0.37, 0.96) |
* Analyses conducted during the evaluation
As safety was assessed at different time points, the results of the comparative safety
analyses should be interpreted cautiously. The safety results are for ‘any’ adverse
event, which includes mild to severe. This makes the comparison difficult to interpret.
9. Clinical Claim
The submission claimed that:
(i) Adalimumab was inferior to infliximab in terms of short term
effectiveness (12 weeks).
Adalimumab was non-inferior in terms of long-term comparative
effectiveness (52 weeks).
Adalimumab was potentially superior to infliximab in terms of
short-term comparative safety and non-inferior to infliximab in
terms of long-term comparative safety.
The PBAC agreed that the pooled odds ratio indirect comparison
suggests adalimumab is inferior to infliximab in achieving a PASI
75 at week 12.
However, the Committee was not satisfied that adalimumab is
non-inferior to infliximab in terms of long-term effectiveness (at
52 weeks).
(ii) Adalimumab was superior to efalizumab in terms of short-term
effectiveness and non- inferior in terms of short-term comparative
safety.
The PBAC was unable to reach a firm conclusion on the relativity of
adalimumab against efalizumab on the basis of the data
presented.
For PBAC’s view, see Recommendation and
Reasons.
10. Economic Analysis
The submission presented a cost minimisation analysis. The
equi-effective doses were estimated as adalimumab 40 mg
subcutaneous injection (1 unit) and infliximab 148 mg intravenous
injection (1.48 units).
11. Estimated PBS Usage and Financial Implication:
The submission estimated a financial cost/year to the PBS of in the
range of $10 – 30 million in year 5.
Recommendations and Reasons:
The PBAC did not accept the submission’s nomination of
infliximab as the primary comparator and efalizumab as a secondary
comparator, noting that efalizumab has the highest market share in
this condition and that infliximab’s listing for psoriasis
become effective only on 1 December 2007. This makes efalizumab the
product most likely to be substituted by adalimumab should it be
recommended for PBS listing. The PBAC however, agreed that a
comparison with infliximab remains useful, particularly if the
sponsor of adalimumab will continue to seek PBS listing on a
cost-effectiveness basis against efalizumab, in which case a
comparison against infliximab would be an appropriate second step
in determining the relativities of all three agents in the
treatment of severe chronic plaque psoriasis.
The Committee agreed with the submission that in comparing the
biological agents in the treatment of chronic plaque psoriasis it
is important to consider both the short term effectiveness (at
around 12 weeks) and the durability of response thereafter. This is
because the PBS subsidy of these agents is currently limited to
those patients who achieve a PASI 75 response after 12 – 16
weeks of treatment and thereafter to patients who maintain this
response at each 6 monthly assessment point.
The PBAC was unable to reach a firm conclusion on the relativity of
adalimumab against efalizumab on the basis of the data presented.
Although the pooled odds ratio of the indirect comparison presented
suggests adalimumab is superior to efalizumab in achieving a PASI
75 after 12 weeks of treatment, no data were presented on the
longer term comparative durability of response to these two agents,
making an overall assessment of relative effectiveness impossible.
Additionally, even if it were accepted that adalimumab is superior
to efalizumab in terms of effectiveness no data were presented on
the cost-effectiveness of using adalimumab instead of
efalizumab.
The PBAC agreed with the sponsor that the pooled odds ratio
indirect comparison suggests adalimumab is inferior to infliximab
in achieving a PASI 75 at week 12. The Committee however found the
claim that adalimumab is non-inferior to infliximab at 52 weeks
more difficult to interpret. This was partly because of the complex
cross-over design of the trials used in this comparison, and
additionally because PBAC considered the most relevant comparison
to be one in which the durability of response in responders at week
12 (or thereabouts) is assessed, rather than overall response from
the beginning to the end of treatment as presented by the
submission, as under the conditions of PBS subsidy only those
patients who respond at week 12 are eligible for continuing
treatment. The Committee noted that the EXPRESS trial reported that
68% of patients (153/225) who had achieved a PASI 75 at week 10
maintained this response to week 50.
Taking into account both the results of short term comparison and
the uncertainty around the longer term comparison, the Committee
was not satisfied that adalimumab is non-inferior to
infliximab.
Overall however, the PBAC rejected the submission on the grounds of
uncertain clinical effectiveness and the resulting uncertain
cost-effectiveness of adalimumab when compared with
efalizumab.
Recommendation
Reject
13. Context for Decision
The PBAC helps decide whether and, if so, how medicines should be
subsidised in Australia. It considers submissions in this context.
A PBAC decision not to recommend listing or not to recommend
changing a listing does not represent a final PBAC view about the
merits of the medicine. A company can resubmit to the PBAC or seek
independent review of the PBAC decision.
14. Sponsor’s Comment
The Sponsor is working with the PBAC to obtain PBS listing for
Humira in chronic plaque psoriasis.




