Quetiapine fumarate, tablets, 25 mg, 100 mg, 200 mg, 300 mg (base), Seroquel®, March 2009
Public summary document for Quetiapine fumarate, tablets, 25 mg, 100 mg, 200 mg, 300 mg (base), Seroquel®, March 2009
Page last updated: 03 July 2009
Public Summary Document
Product:Quetiapine fumarate, tablets, 25 mg, 100
mg, 200 mg, 300 mg (base), Seroquel®
Sponsor:AstraZeneca Pty Ltd
Date of PBAC Consideration: March 2009
1. Purpose of Application
The submission sought an extension to the current Authority
Required (Streamlined) listing for immediate release quetiapine to
include maintenance treatment of bipolar disorder, in combination
with a mood stabiliser, for the prevention of recurrence of manic,
depressive or mixed episodes.
2. Background
The requested indication for the drug had not previously been
considered by the PBAC.
At the June 2000 meeting, the PBAC recommended an authority
required listing for immediate release quetiapine for the treatment
of schizophrenia on a cost-minimisation basis compared with
risperidone.
At the July 2007 meeting, the PBAC recommended extending the
listing for quetiapine immediate release to include the treatment,
as monotherapy, for up to 6 months, of an episode of acute mania
associated with bipolar I disorder. Listing was effective 1
December 2007.
3. Registration Status
Immediate release quetiapine is TGA registered for use in bipolar disorder as:
- Maintenance treatment of bipolar I disorder, as monotherapy or in combination with lithium or sodium valproate, for the prevention of relapse/recurrence of manic, depressive or mixed episodes.
- Treatment of depressive episodes associated with bipolar disorder.
- Treatment of acute mania associated with bipolar I disorder as monotherapy or in combination with lithium or sodium valproate.
4. Listing Requested and PBAC’s View
Authority Required (STREAMLINED)
Maintenance treatment of bipolar I disorder, in combination with a mood stabiliser,
for the prevention of recurrence of manic, depressive or mixed episodes.
For PBAC views see Recommendation and Reasons.
5. Clinical Place for the Proposed Therapy
Bipolar I disorder is a psychiatric condition that is characterised
by the occurrence of one or more manic episodes or mixed episodes.
Often individuals have also had one or more major depressive
episodes.
Treatment to prevent recurrence of mania or depression is termed
maintenance treatment.
Quetiapine in combination with a mood stabiliser offers an
alternative therapy for the maintenance treatment of bipolar 1
disorder.
6. Comparator
The submission nominated olanzapine as the comparator.
The PBAC accepted that olanzapine was the appropriate
comparator.
7. Clinical Trials
The submission presented two randomised trials comparing quetiapine (flexible doses
quetiapine 400-800 mg/day) versus placebo and one randomised trial of olanzapine versus
placebo (Tohen et al 2004) in maintenance treatment of bipolar I disorder (olanzapine
5-20 mg/day flexible dosing) in combination with either lithium or valproate. The
basis of the submission was an indirect comparison of the quetiapine and olanzapine
trial outcomes using placebo as a common comparator.
One of these studies had been published at the time of the submission, as follows:
| Trial/First author | Protocol title | Publication Citation |
|---|---|---|
| Common reference: Placebo | ||
| Olanzapine | ||
| Tohen et al (2004) | Double-blind RCT; Compares the time to mood event of OLZ + LI/VAL vs PLA + LI/VAL for participants in syndromic remission of Bipolar I disorder for up to 18 months. Relapse prevention in bipolar I disorder: 18-month comparison of olanzapine plus mood stabiliser v. mood stabiliser alone. | . British Journal of Psychiatry, 184, 337-345, 2004. |
Abbreviations: RCT=randomised controlled trial; OLZ=olanzapine; LI=lithium; VAL=valproate;
PLA=placebo.
8. Results of Trials
The results of the indirect comparison between quetiapine and olanzapine via placebo as common comparator for the outcome of time to recurrence of mood event are presented in the table below.
| Trial ID | QTP + MS vs PLA + MS HR (95% CI) | OLZ + MS vs PLA + MS HR (95%CI) | QTP + MS vs OLZ + MS Indirect HR (95% CI) | Log rank p value |
|---|---|---|---|---|
| Study 1 a | 0.28 (0.21, 0.37) | - | - | <0.0001 |
| Study 2 a | 0.32 (0.24, 0.42) | - | - | <0.0001 |
| Tohen et al (2004) | - | 0.44 (0.21, 0.91) | - | 0.023 |
| Pooled b | 0.30 (0.25,0.37) | 0.44 (0.21, 0.91) | 0.69 (0.32,1.47) | - |
Abbreviations: OLZ=olanzapine; MS=mood stabiliser (either valproate or lithium); PLA=placebo;
QTP=quetiapine; CI=confidence interval;
a primary outcome in the trial
b pooled using the random effects model
The figure of the forest plot of time to recurrence of mood event across the indirectly
compared randomised trials (hazard ratio) is shown below:
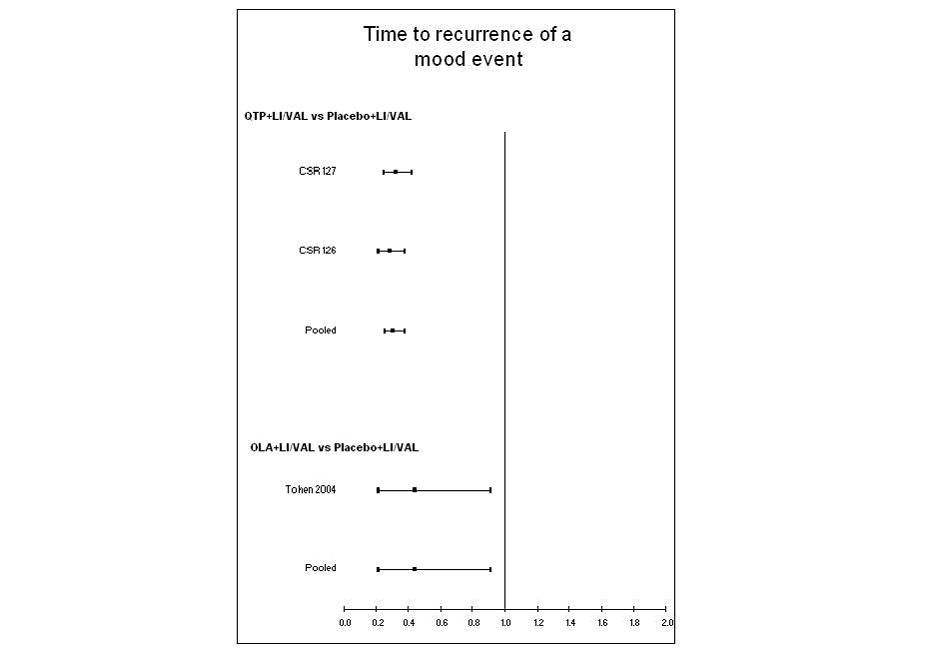
The detail above illustrates results of time to recurrence of any mood event. The
time to recurrence of an event is reported as a hazard ratio (HR) in all three trials.
Time to recurrence of a mood event was significantly longer in patients treated with
quetiapine in combination with valproate or lithium compared to valproate or lithium
alone (pooled HR: 0.30, 95%CI: 0.25, 0.37) representing a 70% reduction in risk. Time
to symptomatic relapse of a mood event was also statistically longer in patients treated
with olanzapine plus valproate or lithium compared to valproate or lithium alone (HR:
0.44, 95% CI: 0.21, 0.91) representing a 56% risk reduction.
Most adverse events reported in quetiapine and olanzapine trials were side effects
that had already been identified in patients treated with these agents for other disorders.
The submission indirectly compared the incidence diarrhoea, insomnia, somnolence,
tremor and weight gain between quetiapine and olanzapine treated patients in the randomised
trials. From the submission’s analyses, patients treated with quetiapine were more
likely to experience somnolence compared to olanzapine treated patients (quetiapine
(QTP) versus olanzapine (OLZ): RR: 11.03 (1.10, 110.37)), the incidence of diarrhoea,
insomnia, tremor and weight gain did not appear to be significantly different.
9. Clinical Claim
The submission claimed quetiapine as non-inferior in terms of
comparative effectiveness and non-inferior in terms of comparative
safety over olanzapine when used in combination with either lithium
or valproate.
While the PBAC considered there was uncertainty in the
interpretation of the data based on an indirect comparison with
placebo as the common reference, it agreed that overall, the
assumption that quetiapine was no worse than olanzapine
reasonable.
10. Economic Analysis
The submission presented a cost-minimisation analysis. The
equi-effective doses estimated as quetiapine 506.8 mg and
olanzapine 8.6 mg/day were considered reasonable.
11. Estimated PBS Usage and Financial Implications
The submission estimated the likely treatment cost of
equi-effective dose of quetiapine and olanzapine to be less than
$10 million per year. The submission estimated potential savings to
the PBS would be approximately $11,000 in Year 5.
For PBAC’s view see Recommendation and
Reasons.
12. Recommendation and Reasons
The PBAC recommended the listing of quetiapine fumarate for the maintenance treatment
of bipolar I disorder, in combination with lithium or sodium valproate on the basis
of cost-minimisation with olanzapine and where the equi-effective doses are quetiapine
506.8 mg per day and olanzapine 8.6 mg per day, i.e., a dose relativity of 58.9:1.
The PBAC accepted that olanzapine was the appropriate comparator. The PBAC considered
the restriction should reflect the Therapeutic Goods Administration (TGA) approved
indication that use of quetiapine be in combination with either lithium or sodium
valproate.
While the PBAC considered there was uncertainty in the interpretation of the data
based on an indirect comparison with placebo as the common reference, it agreed that
overall the assumption that quetiapine was no worse than olanzapine was not unreasonable.
The PBAC did note that the ways in which mood events were defined differed between
the quetiapine and olanzapine trials. Also, the size of the olanzapine trial was smaller
than for quetiapine and thus while there is no significant difference between olanzapine
and placebo this could be due to inadequate power due to small sample size.
The PBAC noted the potential savings may be an overestimate as the submission’s estimate
did not consider any potential switching of patients from other existing PBS-listed
therapies used in combination. The Committee commented that, as quetiapine is listed
as a streamlined authority, it may be difficult to calculate the weighted average
price as the proportion of usage in the current indications and the new indication
will be unknown.
Recommendation:
QUETIAPINE FUMARATE, tablets, 25 mg, 100 mg, 200 mg, 300 mg (base).
Extend the listing as follows to include:
Restriction:
Authority Required (STREAMLINED)
Maintenance treatment of bipolar I disorder, in combination with lithium or sodium
valproate.
Max. Qty: 60 (25 mg, 200 mg and 300 mg), 90 (100 mg)
Repeats: 5
13. Context for Decision
The PBAC helps decide whether and, if so, how medicines should be
subsidised in Australia. It considers submissions in this context.
A PBAC decision not to recommend listing or not to recommend
changing a listing does not represent a final PBAC view about the
merits of the medicine. A company can resubmit to the PBAC or seek
independent review of the PBAC decision.
14. Sponsor’s Comment
AstraZeneca welcomes the PBAC recommendation for Seroquel to be
listed on the PBS, to provide access to an additional treatment
option for patients with bipolar I disorder.




