Quetiapine fumarate, tablets, 25 mg, 100 mg, 200 mg, 300 mg (base), Seroquel®, March 2009
Public summary document for Quetiapine fumarate, tablets, 25 mg, 100 mg, 200 mg, 300 mg (base), Seroquel®, March 2009
Page last updated: 17 July 2009
Public Summary Document
Product:Quetiapine fumarate, tablets, 25 mg, 100
mg, 200 mg, 300 mg (base), Seroquel®
Sponsor: AstraZeneca Pty Ltd
Date of PBAC Consideration: March 2009
1. Purpose of Application
To submission sought an extension to the current Authority required
(Streamlined) listing for immediate release quetiapine to include
treatment of a patient with depressive episodes associated with
bipolar disorder.
2. Background
The requested indication had not previously been considered by the
PBAC.
At the June 2000 meeting, the PBAC recommended an authority
required listing for immediate release quetiapine for the treatment
of schizophrenia on a cost-minimisation basis compared with
risperidone.
At the July 2007 meeting, the PBAC recommended extending the
authority required PBS listing for quetiapine immediate release to
include the treatment, as monotherapy, for up to 6 months, of an
episode of acute mania associated with bipolar I disorder. Listing
was effective 1 December 2007.
3. Registration Status
Immediate release quetiapine is TGA registered for use in bipolar disorder as:
- Maintenance treatment of bipolar I disorder, as monotherapy or in combination with lithium or sodium valproate, for the prevention of relapse/recurrence of manic, depressive or mixed episodes.
- Treatment of depressive episodes associated with bipolar disorder.
- Treatment of acute mania associated with bipolar I disorder as monotherapy or in combination with lithium or sodium valproate.
4.Listing Requested and PBAC’s View
Authority Required (STREAMLINED)
Depressive episodes associated with bipolar disorder.
For PBAC views, see Recommendation and Reasons.
5. Clinical Place for the Proposed Therapy
Bipolar disorders are characterised by episodic depressions and
elevations of mood.
Quetiapine would provide an alternative therapy for acute
depressive episodes.
6. Comparator
The submission nominated olanzapine as the main comparator.
The PBAC considered that olanazapine was not the appropriate
comparator as olanzapine is not TGA registered or PBS listed for
acute treatment of depressive episodes in bipolar disorder.
For PBAC’s view, see Recommendations and
Reasons.
7. Clinical Trials
The scientific basis of the submission was an indirect comparison of quetiapine versus
olanzapine using placebo as a common comparator. The submission presented four (4)
randomised trials comparing quetiapine 300 mg daily and 600 mg daily (fixed dose)
BOLDER I and II, EMBOLDEN I and EMBOLDEN II with placebo and one trial of olanzapine
(flexible dosing) Tohen et al 2003,versus placebo in patients with bipolar depression.
Although the quetiapine trials enrolled patients with both bipolar I and bipolar II
disorders, only results of efficacy and safety in the subgroup of patients with bipolar
I disorder with acute depressive episodes were indirectly compared between olanzapine
and quetiapine. This was because only bipolar I patients were enrolled in the olanzapine
trial.
The studies that had been published at the time of the submission, are as follows:
| Trial/First author | Protocol title/publication citation | Publication citation |
|---|---|---|
| Common reference: placebo | ||
| Quetiapine | ||
| BOLDER I | ||
| Calabrese et al | A randomized, double-blind, placebo-controlled trial of quetiapine in the treatment of bipolar I or II depression. | American Journal of Psychiatry 162:1351-1360, 2005. |
| Hirschfeld et al | Quetiapine in the treatment of anxiety in patients with bipolar I or II depression: A secondary analysis from a randomized, double-blind, placebo-controlled study. | Journal of Clinical Psychiatry 67:355-362, 2006. |
| Cookson et al | Number needed to treat and time to response/remission for quetiapine monotherapy efficacy in acute bipolar depression: Evidence from a large, randomized, placebo-controlled study. | International Clinical Psychopharmacology 22:93-100, 2007. |
| Endicott et al | A randomized, double-blind, placebo-controlled study of quetiapine in the treatment of bipolar I and II depression: Improvements in quality of life. | International Clinical Psychopharmacology 22:29-37, 2007. |
| Vieta E et al | Quetiapine monotherapy in the treatment of patients with bipolar I or II depression and a rapid-cycling disease course: A randomized, double-blind, placebo-controlled study. | Bipolar Disorders 9:413-425, 2007. |
| Gajwani et al | Update on quetiapine in the treatment of bipolar disorder: Results from the BOLDER studies. | Neuropsychiatric Disease and Treatment 3:847-853, 2007. |
| Weisler et al | Efficacy of quetiapine monotherapy for the treatment of depressive episodes in bipolar I disorder: A post hoc analysis of combined results from 2 double-blind, randomized, placebo-controlled studies. [note: combined analysis with BOLDER II] | Journal of Clinical Psychiatry 69:769-782, 2008. |
| BOLDER II | ||
| Thase et al 2006 | Efficacy of quetiapine monotherapy in bipolar I and II depression: A double-blind, placebo-controlled study (the BOLDER II study). | Journal of Clinical Psychopharmacology 26:600-609, 2006. |
| Gajwani et al 2007 | Update on quetiapine in the treatment of bipolar disorder: Results from the BOLDER studies. | Neuropsychiatric Disease and Treatment 3:847-853, 2007. |
| Gajwani 2007 | BOLDER II study of quetiapine therapy for bipolar depression. | Future Neurology 2:373-377, 2007. |
| Weisler et al | Efficacy of quetiapine monotherapy for the treatment of depressive episodes in bipolar I disorder: A post hoc analysis of combined results from 2 double-blind, randomized, placebo-controlled studies. [note: combined analysis with BOLDER I] | Journal of Clinical Psychiatry 69:769-782, 2008. |
| Olanzapine | ||
| Tohen et al 2003 | Efficacy of olanzapine and olanzapine-fluoxetine combination in the treatment of bipolar I depression. | Archives of General Psychiatry 60:1079-1088, 2003. |
| Shi et al | Effects of olanzapine alone and olanzapine/fluoxetine combination on health-related quality of life in patients with bipolar depression: secondary analyses of a double-blind, placebo-controlled, randomized clinical trial. | Clinical Therapeutics 26:125-134, 2004. |
| Keck et al | Analyses of treatment-emergent mania with olanzapine/fluoxetine combination in the treatment of bipolar depression. | Journal of Clinical Psychiatry 66:611-616, 2005. |
| Keck et al | A 24-week open-label extension study of olanzapine-fluoxetine combination and olanzapine monotherapy in the treatment of bipolar depression. | Journal of Clinical Psychiatry 67:798-806, 2006. |
| Williamson et al | Clinical relevance of depressive symptom improvement in bipolar I depressed patients. | Journal of Affective Disorders 92:261-266, 2006. |
| Dube et al | Onset of antidepressant effect of olanzapine and olanzapine/fluoxetine combination in bipolar depression. | Bipolar Disorders 9: 618-627, 2007. |
| Tohen M et al | Effect of comorbid anxiety on treatment response in bipolar depression. | Journal of Affective Disorders 104:137-146, 2007. |
| Amsterdam & Shults | Comparison of fluoxetine, olanzapine, and combined fluoxetine plus olanzapine initial therapy of bipolar type I and type II major depression - Lack of manic induction. | Journal of Affective Disorders 87:121-130, 2005. |
8. Results of Trials
The primary outcome of the quetiapine and olanzapine trials was change in total Montgomery-Asberg
Depression Rating Scale (MADRS) scores between baseline and Week 8 of the trials.
The results of the submission’s indirect comparison of change in MADRS total score
between baseline and Week 8 of quetiapine 300 mg daily versus olanzapine treatment
in bipolar I patients using mixed effects model repeat measures (MMRM) analyses indicated
that there was no significant difference between quetiapine and olanzapine for the
change in total MADRS score between baseline and Week 8.
The pooled efficacy comparisons of change in total MADRS score from baseline to Week
8 are summarised in the table below. Additional analyses, conducted during the evaluation,
of the efficacy of quetiapine 300 mg versus quetiapine 600 mg and in bipolar I versus
bipolar II patients are also presented in the table below in italics.
| Comparisons | LOCF WMD (95% CI) | MMRM WMD (95% CI) |
|---|---|---|
| QET 300mg vs placebo (BOLDER I, BOLDER II, EMBOLDEN I AND EMBOLDEN II) | ||
| All patients | -4.7 (-6.1, -3.2) N: 811 vs 580 | -4.2 (-6.2, -2.3) N: 573 vs 370 |
| Bipolar I | -5.1 (-7.2, -2.9) N:528 vs 376 | -5.0 (-7.52, -2.48) N: 362 vs 226 |
| Bipolar II | -4.1 (-5.9, -2.2) N: 283 vs 204 | -3.7 (-5.6, -1.8) N: 211 vs 144 |
| QET 600mg vs Placebo (BOLDER I, BOLDER II, EMBOLDEN I AND EMBOLDEN II) | ||
| All patients | -4.6 (-5.8, -3.3) N: 816 vs 580 | -4.5 (-6.5, -2.6) N: 544 vs 370 |
| Bipolar I | -5.4 (-7.9, -2.8) N: 527 vs 376 | -5.3 (-8.2, -2.4) N:352 vs 226 |
| Bipolar II | -3.1 (-5.0, -1.3) N: 289 vs 205 | -3.6 (-5.5, -1.7) N: 192 vs 144 |
| Olanzapine vs placebo (Tohen 2003) | ||
| Bipolar I | - | -3.1 (-5.2, -1.0) N: 179 vs 145 |
| QET 600mg vs QET 300mg | ||
| All patients | - | -0.34 (-1.53, 0.85) N:544 vs 573 |
| Bipolar I | - | -0.46 (-1.87, 0.94) N: 352 vs 362 |
| Bipolar II | - | -0.20 (-1.91, 1.51) N:192 vs 211 |
| Bipolar I versus bipolar II | ||
| Quetiapine 300mg QD | - | -1.10 (-2.68, 0.47) N: 362 vs 211 |
| Quetiapine 600mg QD | - | -1.36 (-3.18, 0.46) N: 352 vs 192 |
Italics indicate additional analyses conducted during the evaluation, bold typography
indicates statistically significant results.
Abbreviations: LOCF=last observation carried forward; OC=observed cases analysis (whereby
change from baseline was calculated at each assessment); MMRM=mixed effects model
repeat measures; MADRS=Montgomery-Asberg Depression Rating Scale; QET=quetiapine;
WMD=weighted mean difference.
Both quetiapine 300 mg and 600 mg daily significantly reduced total MADRS scores from
baseline to Week 8 compared with placebo treatment. The effects are numerically greater
in patients diagnosed with bipolar I disorder compared to bipolar II disorder; however,
the differences are not statistically significant. Analyses using mixed effects model
repeat measures (MMRM) and last observation carried forward (LOCF) appear to reach
similar conclusions.
The mean change from baseline in total MADRS score (MMRM analysis) for the indirect
comparison of quetiapine versus olanzapine is shown in the figure below:
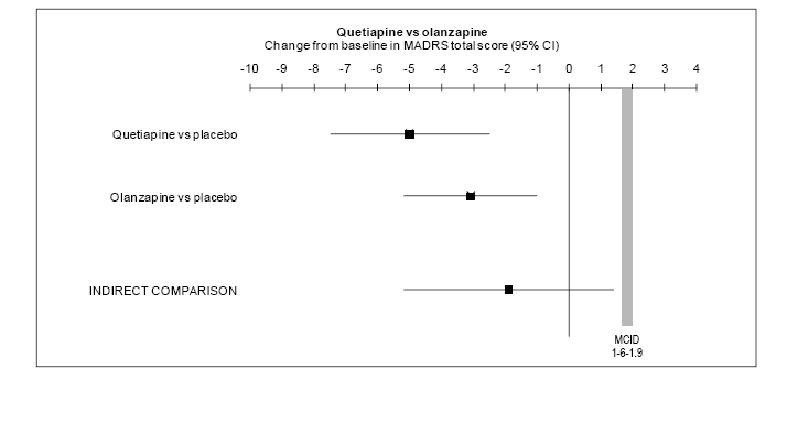
The result of the weight adjusted mean difference in change of MADRS total scores
from baseline to week 8 for the indirect comparison of quetiapine 300 mg/day versus
olanzapine (average daily dose 9.7 mg) via placebo as the common comparator is -1.9
(95% CI: -5.2, 1.4). This is within the non-inferiority threshold nominated by the
submission (minimal clinically important difference (MCID) threshold for MADRS between
1.6-1.9).
The following adverse events were reported significantly more for both quetiapine
and olanzapine than for placebo: somnolence, weight gain, increased appetite, headache,
dry mouth, insomnia, dyspepsia and nausea.
9. Clinical Claim
The submission claimed quetiapine is non-inferior in terms of
comparative effectiveness and equivalent in terms of comparative
safety over olanzapine.
The PBAC accepted this claim, however, because olanzapine was not
the appropriate comparator for the cost-minimisation analysis
presented, this could not be an acceptable basis for the
listing.
10.Economic Analysis
The submission presented a cost minimisation analysis. The
submission estimated that quetiapine 270.4 mg and olanzapine 9.7 mg
daily are equi-effective. However, the additional comparisons
conducted during the evaluation demonstrated that there were no
significant differences between the outcomes of change in MADRS
total score from baseline to Week 8 for patients treated with
quetiapine 300 mg daily and quetiapine 600 mg daily. (See Results
of Trials: Table with pooled efficacy comparison of change in total
MADRS score from baseline to week 8).
Therefore, it might be possible that olanzapine 9.7 mg might also
be equivalent to quetiapine 503.9 mg (mean median dose from the
quetiapine 600 mg arm of the trials), hence, the dose relativity of
olanzapine : quetiapine in bipolar depression may be between the
ratios of 1:27.9 to 1:51.9, i.e., the equi-effective dose may be
underestimated.
11. Estimated PBS Usage and Financial Implications
The submission estimated the financial savings per year to the PBS
to be < $10 million per year in Year 5. The submission’s
estimate was considered to be an overestimate of the savings.
12. Recommendation and Reasons
The main issue of concern to the PBAC was the choice of comparator.
Olanzapine is not TGA registered or PBS listed for acute treatment
of depressive episodes in bipolar disorder. The current PBS listing
for olanzapine for maintenance treatment of bipolar 1 disorder does
not encompass acute treatment of bipolar depression. The PBAC noted
therapeutic guidelines indicated that in de novo depression the
options for treatment include a mood stabiliser (lithium, sodium
valproate, carbamazepine, lamotrigine, olanzapine, risperidone and
quetiapine) alone or in combination with an antidepressant. Of
these, only lithium, carbamazepine and valproate are PBS available
for these patients, being unrestricted benefits, and quetiapine
appeared to be the only agent with a specific TGA listing for the
indication (acute depression). Further, while it was accepted
olanzapine is being prescribed for depressive episodes, the results
of the expert survey indicated that olanzapine is not the treatment
that prescribers would most replace with quetiapine.
The indirect comparison presented indicated there was no
significant difference between quetiapine and olanzapine for the
change in total Montgomery-Asberg Depression Scale (MADRS) score
between baseline and week 8. The PBAC noted the placebo response
rate was similar for the two drugs, and hence the direct comparison
appeared to be valid. Further, most adverse events reported in
quetiapine and olanzapine trials were side effects that have
already been identified in patients treated for other
disorders.
Therefore, the PBAC accepted the clinical claim that quetiapine
could be described as non-inferior in terms of comparative
effectiveness and in terms of comparative safety over olanzapine in
the treatment of depressive episodes associated with bipolar
disorder. However, because olanzapine was not the appropriate
comparator for the cost-minimisation analysis presented, this could
not be an acceptable basis for listing.
The PBAC did comment that the exclusive consideration of quetiapine
300 mg daily outcomes in the cost-minimisation analysis
underestimates the equi-effective dose of quetiapine versus
olanzapine, which could be quetiapine 503.9 mg:9.7 mg olanzapine as
opposed to 270.4 mg:9.7 mg or very likely, a figure somewhere in
between.
With respect to an appropriate cost-effectiveness analysis to
justify a listing, the PBAC noted the pre-PBAC response stated data
were available for lithium and paroxetine from the two EMBOLDEN
studies.
The PBAC rejected listing on the basis the main comparator was
inappropriate.
The PBAC noted there is clearly a mismatch between clinical
guidelines/common use, TGA registration and current PBS listings
and there is a need to get clinical consensus in order for
companies to present appropriate data for TGA/PBS. It was
considered this issue needs to be addressed and may require a
stakeholder meeting to progress the matter.
The PBAC noted that the submission meets the criteria for an
Independent Review.
Recommendation
Reject
13. Context for Decision
The PBAC helps decide whether and, if so, how medicines should be
subsidised in Australia. It considers submissions in this context.
A PBAC decision not to recommend listing or not to recommend
changing a listing does not represent a final PBAC view about the
merits of the medicine. A company can resubmit to the PBAC or seek
independent review of the PBAC decision.
14. Sponsor’s Comment
AstraZeneca understands the PBAC’s position. There is a
clinical need for quetiapine in this population and AstraZeneca is
committed to working with the PBAC to reach a satisfactory
outcome.




