Docetaxel, injection set containing 1 single use vial concentrate for I.V. infusion 20 mg (anhydrous) in 0.5 mL and 1 single use vial solvent 1.5 mL and injection set containing 1 single use vial concentrate for I.V. infusion 80 mg (anhydrous) in 2 mL and 1 single use vial solvent 6 mL, Taxotere®, November 2009
Public Summary Document for Docetaxel, injection set containing 1 single use vial concentrate for I.V. infusion 20 mg (anhydrous) in 0.5 mL and 1 single use vial solvent 1.5 mL and injection set containing 1 single use vial concentrate for I.V. infusion 80 mg (anhydrous) in 2 mL and 1 single use vial solvent 6 mL, Taxotere®, November 2009
Page last updated: 05 March 2010
Public Summary Document
Product: Docetaxel, injection set containing 1
single use vial concentrate for I.V. infusion 20 mg (anhydrous) in
0.5 mL and 1 single use vial solvent 1.5 mL and injection set
containing 1 single use vial concentrate for I.V. infusion 80 mg
(anhydrous) in 2 mL and 1 single use vial solvent 6 mL,
Taxotere®
Sponsor: Sanofi Aventis Australia Pty Ltd
Date of PBAC Consideration: November 2009
1. Purpose of Application
The submission requested to extend the current authority required
listing for docetaxel to include adjuvant treatment of operable
breast cancer in combination with cyclophosphamide.
2. Background
Docetaxel has not previously been considered by the PBAC for
adjuvant treatment of operable breast cancer in combination with
cyclophosphamide.
3. Registration Status
The TGA registration for docetaxel was extended on 21 August 2009
to include the adjuvant treatment of operable breast cancer with a
primary tumour of greater than or equal to 1 cm and less than 7 cm,
in combination with cyclophosphamide; Doxorubicin and
cyclophosphamide followed by docetaxel in combination with
trastuzumab (AC-TH) is indicated for the adjuvant treatment of
patients with operable breast cancer whose tumours overexpress HER2
and docetaxel in combination with carboplatin and trastuzumab (TCH)
is indicated for the adjuvant treatment of patients with operable
breast cancer whose tumours overexpress HER2.
Docetaxel was first TGA registered on 6 February 1996, and is also
registered for the following indications:
Breast cancer:
- Locally advanced or metastatic breast cancer in whom previous chemotherapy has failed;
- In combination with capecitabine for the treatment of patients with locally advanced or metastatic breast cancer after failure of prior anthracycline containing chemotherapy;
- In combination with doxorubicin and cyclophosphamide is indicated for the adjuvant treatment of patients with node positive breast cancer.
- In combination with trastuzumab for the treatment of patients with metastatic breast cancer whose tumours overexpress HER2 and who previously have not received chemotherapy for metastatic disease.
Non-small cell lung cancer:
Locally advanced or metastatic non-small cell lung cancer,
including those who have failed platinum based chemotherapy.
Ovarian cancer:
Metastatic carcinoma of the ovary after failure of first line or
subsequent chemotherapy.
Prostate cancer:
Androgen independent (hormone refractory) prostate cancer.
Head and neck cancer:
Locally advanced, squamous cell carcinoma.
4. Listing Requested and PBAC’s View
Authority Required
Adjuvant treatment of operable breast cancer in combination with
cyclophosphamide.
For PBAC’s view see Recommendation and
Reasons.
5. Clinical Place for the Proposed Therapy
Breast cancer is the most notifiable cancer for women in Australia,
accounting for more than one in four of all female cancers, and the
most common cause of cancer-related death.
In patients with early breast cancer, the cancer is operable and is
restricted to the breast. The exception is in women with
‘node positive’ disease, where the cancer involves the
lymph nodes. The primary treatment in most situations is surgery.
Treatment given subsequent to surgery is referred to as
‘adjuvant’ and aims to achieve total disease control.
Doxorubicin (an anthracycline) plus cyclophosphamide (AC) is the
most common adjuvant treatment of breast cancer. However, there are
long term cardiotoxicity and bone marrow toxicity risks with
anthracycline use.
Docetaxel plus cyclophosphamide (TC) regimen would provide an
alternative non-anthracycline adjuvant treatment of operable breast
cancer.
6. Comparator
The submission nominated the chemotherapy regimen of doxorubicin
plus cyclophosphamide (AC) as the main comparator.
The PBAC considered that the comparator AC was appropriate but that
a wider range of therapies might be utilised in clinical practice
in this patient population.
7. Clinical Trials
The submission presented one randomised trial comparing docetaxel (75 mg/m2) plus cyclophosphamide (600 mg/m2) with doxorubicin (60 mg/m2) plus cyclophosphamide (600 mg/m2), administered intravenously every 21 days for four cycles, in patients with operable
breast cancer as adjuvant treatment, over a follow-up of seven years.
The key trials published at the time of submission are shown in the table below:
| Trial ID/First author | Full Title | Publication citation |
|---|---|---|
| Full report of 5-year follow-up | ||
| Jones et al. 2006 | Phase III trial comparing doxorubicin plus cyclophosphamide as adjuvant therapy for operable breast cancer. | Journal of Clinical Oncology 24(34):5381-5387 |
| Abstract reports of 5-year follow-up | ||
| Jones et al. 2001 | Preliminary results of a prospective randomized trial of adjuvant chemotherapy for patients with stage I-III operable, invasive breast cancer comparing four courses of Adriamycin/cyclophosphamide (AC) to four courses of Taxotere/cyclophosphamide (TC). | Proceedings of the American Society of Clinical Oncology 2001, Vol 20, Part 1, p 33a, Abstract Number 128. 37th Annual Meeting of ASCO, San Francisco (May 12-15, 2001) |
| Jones et al. 2003 | Three year results of a prospective randomized trial of adjuvant chemotherapy for patients (pts) with stage I-III operable, invasive breast cancer comparing 4 courses of doxorubicin/cyclophosphamide (AC) to 4 course of docetaxel/cyclophosphamide (TC). | Proc Am Soc Clin Oncology 22:15, 2003 (abstract 59) |
| Jones et al. 2005 | Final analysis: TC (docetaxel/cyclophosphamide, 4 cycles) has a superior disease-free survival compared to standard AC (doxorubicin/cyclophosphamide) in 1016 women with early stage breast cancer. | Breast Cancer Res Treat 2005; 94(Suppl 1): S20, Abs: 40 Presented at the 28th Annual San Antonio Breast Cancer Symposium, December 8-11, 2005 |
| Full report of 7-year follow-up | ||
| Jones et al. 2009 | Docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7-year follow-up of US Oncology Adjuvant trial 9735. | Journal of Clinical Oncology 27(8): 1177-1183 |
| Abstract report of 7-year follow-up | ||
| Jones et al. 2007 | Extended follow-up and analysis by age of the US Oncology Adjuvant trial 9735: docetaxel/cyclophosphamide is associated with an overall survival benefit compared to doxorubicin/cyclophosphamide and is well tolerated in women 65 or older. | Breast Cancer Res Treat 2007; 106(Suppl 1): S5, Abs: 12. 30th Annual San Antonio Breast Cancer Symposium (SABCS), San Antonio, TX (Dec 2007) |
ASCO = American Society of Clinical Oncology; TX = Texas
8. Results of Trials
The submission conducted analyses of disease-free survival and overall survival at
5 and 7 years follow up.
Five-year analyses
The disease-free survival (primary outcome) and overall survival (secondary outcome)
at 5 years are presented in the figures below.
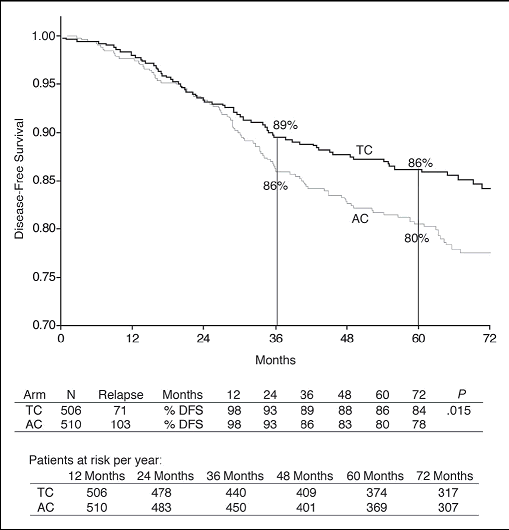
Disease-free survival at 5 years
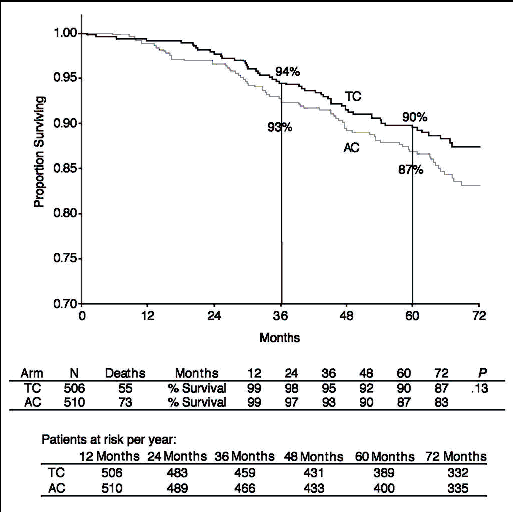
Overall survival at 5 years
TC = docetaxel plus cyclophosphamide; AC = doxorubicin plus cyclophosphamide
The disease-free survival rate for patients taking TC was 86% compared with 80% for
patients receiving AC at 5 years (hazard ratio (HR) = 0.67, 95% confidence intervals
(CI) 0.50, 0.94, p=0.015).
The overall survival rate for women treated with TC was 90% compared with 87% for
women treated with AC (HR 0.76, 95% CI 0.52, 1.1, p = 0.13) at the 5-year follow-up.
There were no statistically significant differences in overall survival between the
two treatment arms at 5 years.
Seven-year analyses
The submission claimed that overall survival became a primary outcome in the seven-year
follow-up analysis, after the trial protocol was amended. The PBAC noted that the
protocol amendment did not specify that overall survival became a primary outcome
in the 7-year analysis. Therefore, the Committee considered that overall survival
should be treated as a secondary outcome, but was the more informative analysis.
The disease-free survival and overall survival curves are presented below.
Disease-free survival at 7-year follow-up
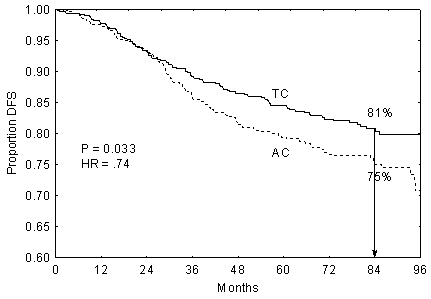
| At Risk | TC | 506 | 481 | 442 | 410 | 378 | 349 | 320 | 195 |
| AC | 510 | 483 | 449 | 405 | 372 | 343 | 303 | 194 |
Overall survival at 7-year follow-up
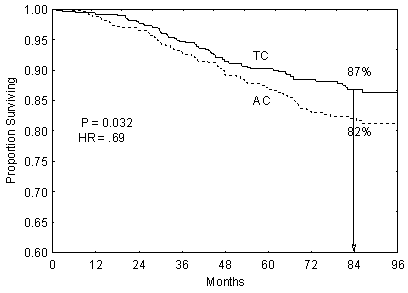
| At Risk | TC | 506 | 487 | 461 | 434 | 398 | 371 | 344 | 207 |
| AC | 510 | 488 | 464 | 438 | 407 | 375 | 327 | 210 |
TC = docetaxel + cyclophosphamide; AC = doxorubicin + cyclophosphamide; HR = hazard
ratio
The disease-free survival rate for women treated with TC was 81% compared with 75%
for women treated with AC (HR 0.74, 95% CI 0.56, 0.98, p = 0.033) at 7-year follow-up.
The PBAC noted that the survival rate was 87% for patients treated with TC and 82%
for those in the AC arm (HR = 0.69, 95% CI 0.50, 0.97, p=0.032; 58 vs 24 deaths),
with a reduction in risk of relapse of approximately 20%, which was similar to that
seen with second generation chemotherapy protocols such as 5-fluorouracil, epirubicin
and cyclophosphamide (FE(100)C) x 6 cycles.
The trial results showed that TC patients experienced significantly more Grade 1 and
2 oedema, myalgia and arthralgia (p<.01), whereas AC patients had more Grade 1 to
4 nausea and vomiting (p<.01). More fever and neutropenia was observed with TC (25
patients, 5%) compared with AC (13 patients, 2.5%, p=0.07). Two patients died while
receiving TC (one unrelated cardiac death and one death with sepsis and neutropenia).
No patients died during the treatment with AC. It appears that TC is associated with
increased short-term toxicities compared with AC.
There were three late deaths without relapse in the AC arm and the submission claimed
that these three deaths were likely to be related to treatment. One of the deaths
was from cardiomyopathy and congestive heart failure, and two died of complications
related to myelodysplasia and myelofibrosis respectively. As there was no formal comparison
of cardiac function at each assessment visit between treatment arms in the trial,
the causal relation between AC treatment and these deaths is uncertain.
The submission provided additional data on potential safety concerns beyond those
identified in the clinical trial. The submission stated that the Periodic Safety Update
Report, which monitored the post-marketing safety of docetaxel over 15 years, did
not identify new safety issues for docetaxel. The submission claimed that the use
of anthracycline increases the risk of cardio-toxicity, congestive heart failure and
bone marrow toxicity. Long term cardio-toxicity of doxorubicin has been confirmed
in the Product Information of doxorubicin.
For PBAC’s comments, see Recommendation and Reasons.
9. Clinical Claim
The submission described docetaxel in combination with
cyclophosphamide as superior in terms of comparative effectiveness
and non-inferior in terms of comparative safety over doxorubicin in
combination with cyclophosphamide.
For PBAC’s view, see Recommendation and
Reasons.
10. Economic Analysis
A modelled stepped economic evaluation was presented. The model
estimated the proportion of patients (at monthly intervals) in both
TC and AC arms who are alive, and who have recurrent disease (local
or distant) based on Kaplan-Meier curves. Differences in mean
survival between TC and AC were estimated as the area under the
Kaplan-Meier curve for overall survival.
Within the trial period of 7 years, a curve of relapse was derived
from the disease-free survival (DFS) curve. After the trial period
of 7 years, it was assumed that no patients will relapse. Time
spent in a health state of being alive or of relapse, which was
estimated from the area under the Kaplan-Meier curves, was
transformed to quality adjusted life years by using utility values
generated in an Australian utility valuation study.
The time horizon in the modelled economic evaluation was 35
years.
The trial based cost-effectiveness ratio (including wastage) was in
the range $45,000 - $75,000 per QALY gained.
The modelled cost-effectiveness ration (including wastage) was less
than $15,000 per QALY gained.
11. Estimated PBS Usage and Financial Implications
The likely number of patients per year was estimated to be less
than 10,000 in Year 5. The submission’s estimate was
considered to be uncertain.
The financial cost per year to the PBS was estimated to be less
than $10 million in Year 5. The submission’s estimate was
considered to be uncertain.
12. Recommendation and Reasons
The PBAC recommended listing of docetaxel on the PBS for the
adjuvant treatment of operable breast cancer in combination with
cyclophosphamide on the basis of an uncertain but acceptable
cost-effectiveness ratio compared with the chemotherapy regimen
doxorubicin with cyclophosphamide (AC) given every 21 days for 4
cycles. The PBAC advised that it would be appropriate for the
Pricing Authority to apply its usual methodology when considering
the price for this extension to listing, which has substantial
financial implications.
The PBAC considered that the comparator AC was appropriate but that
a wider range of therapies might be utilised in clinical practice
in this patient population, as acknowledged in the sponsor’s
Pre-Sub-Committee Response. However, the PBAC noted that TC has a
survival rate at 7 years of 87% compared with 82% for AC [HR 0.69,
95% CI 0.50, 0.97, p = 0.032], a reduction in risk of relapse of
approximately 20%, which is similar to that seen with second
generation chemotherapy protocols such as FE(100)C x 6 cycles.
However, the cost-effectiveness of TC versus second generation
protocols such as FE(100)C is unknown. Therefore, the PBAC
considered that the number of cycles of TC should be limited to 4,
consistent with the number of cycles administered in the trial,
which may decrease the uncertainty associated with the cost
effectiveness.
The PBAC noted that the list of side effects in the trial was
incomplete and did not include common side effects of docetaxel
such as peripheral neuropathy, nail changes, constipation, skin
eruptions and fatigue. The PBAC considered that the rates of
febrile neutropenia were underestimated and that this may have been
due to the fact that the use of prophylactic antibiotics was
permitted in the trial. The PBAC considered that the disutility of
the side effects from docetaxel was underestimated and that the
ICER was therefore uncertain and likely to be higher.
Further, it was assumed that the cost of treating local recurrence
is the same as that of distant recurrence. However, the two types
of recurrence are likely to have different survival, quality of
life and treatment costs. The PBAC noted there is a higher
proportion of local relapse in the AC arm than in the TC arm.
However, the submission uses an average value across both treatment
arms to estimate the split of local and distant recurrence in order
to derive the weighted utility for recurrence. The PBAC considered
that this was not appropriate and was likely to bias the result in
favour of TC, as TC has a higher distant recurrence than AC and
distant recurrence has a lower utility than local recurrence.
The PBAC agreed with the ESC that none of the four extrapolation
methods presented in the submission were conservative and that the
incremental cost-effectiveness ratios calculated represented
best-case scenarios. Extrapolation from a trial-duration of 7 years
to a time horizon of 35 years had driven the model from an
incremental cost of between $45,000 – $75,000/QALY to less
than $15,000/QALY. However, the PBAC considered that the duration
of extrapolation meant that the results are subject to considerable
uncertainty and that the ICER could be higher than those estimated
in the submission, although unlikely to be higher than the
trial-based ICER of between $45,000 – $75,000/QALY which was
high but acceptable.
Recommendation:
DOCETAXEL, injection set containing 1 single use vial concentrate
for I.V. infusion, 20 mg (anhydrous) in 0.5 mL and 1 single use
vial solvent 1.5 mL and injection set containing 1 single use vial
concentrate for I.V. infusion, 80 mg (anhydrous) in 2 mL and 1
single use vial solvent 6 mL
Extend the current restriction to include:
Restriction:
Authority Required
Adjuvant treatment of operable breast cancer in combination with cyclophosphamide
Maximum quantity:
2 (20 mg in 0.5mL)
1 (80mg in 2mL)
Repeats: 0
13. Context for Decision
The PBAC helps decide whether and, if so, how medicines should be
subsidised in Australia. It considers submissions in this context.
A PBAC decision not to recommend listing or not to recommend
changing a listing does not represent a final PBAC view about the
merits of the medicine. A company can resubmit to the PBAC or seek
independent review of the PBAC decision.
14. Sponsor’s Comment
Sanofi-aventis welcomes the PBAC’s decision to recommend PBS
listing of docetaxel in combination with cyclophosphamide for use
as adjuvant chemotherapy treatment of patients with operable breast
cancer.




