Everolimus, tablet, 5 mg and 10 mg, Afinitor®, November 2009
Public Summary Document for Everolimus, tablet, 5 mg and 10 mg, Afinitor®, November 2009
Page last updated: 19 March 2010
PDF printable version for Everolimus, tablet, 5 mg and 10 mg, Afinitor® (PDF 64 KB)
Public Summary Documents
Product: Everolimus, tablet, 5 mg and 10 mg,
Afinitor®
Sponsor: Novartis Pharmaceuticals Australia Pty
Ltd
Date of PBAC Consideration: November 2009
1. Purpose of Application
The submission sought an authority required PBS listing for the
treatment of a patient with Stage IV clear cell variant renal cell
carcinoma (RCC) after failure of sunitinib or sorafenib.
2. Background
This drug has not previously been considered by the PBAC for this
indication.
3. Registration Status
Everolimus (Afinitor) was designated an orphan drug by the TGA on
17 July 2008.
Everolimus tablets 5 mg and 10 mg were TGA registered on 6 August
2009 for the treatment of patients with advanced renal cell
carcinoma after failure of treatment with sorafenib or
sunitinib.
4. Listing Requested and PBAC’s View
Authority required
Treatment, as the sole PBS-subsidised therapy, of a patient with
Stage IV clear cell variant renal cell carcinoma after failure of
treatment with sorafenib or sunitinib.
Authority required (grandfather)
Treatment, as the sole PBS-subsidised therapy, of a patient with
Stage IV clear cell variant renal cell carcinoma who was eligible
under the above criteria and was receiving treatment with
everolimus prior to (insert LISTING DATE).
NOTE:
Patients should continue everolimus treatment until the first
occurrence of radiologically documented disease progression
according to RECIST criteria.
For PBAC’s view, see Recommendation and
Reasons.
5. Clinical Place for the Proposed Therapy
Renal cell carcinoma (RCC) is a form of kidney cancer that arises
from the cells of the renal tubule. The management and prognosis of
a patient with RCC is determined by the stage of the disease.
Surgery is the only curative treatment option for localised RCC.
Most patients are diagnosed with advanced RCC which is often
refractory to treatment and associated with a poor prognosis.
Currently, only sunitinib is PBS listed for this indication.
Everolimus would be a new treatment option for patients with
advanced RCC who have failed sunitinib.
6. Comparator
Appropriately, the submission nominated placebo for best supportive
care as the main comparator as there are no PBS-subsidised
second-line treatment alternatives upon disease progression after
first-line treatment with sunitinib.
7. Clinical Trials
The submission presented one randomised trial, RECORD-1, comparing everolimus, 10
mg per day orally, with placebo in patients with Stage IV clear cell renal cell carcinoma
with Karnofsky performance score of at least 70 and having demonstrated progression
on or within six months of treatment with sunitinib or sorafenib. Crossover to everolimus
for placebo patients was allowed after progression.
The trial published at the time of submission is shown in the table below. This publication
used the second interim analysis data cut-off of 15 October 2007.
| Trial ID/First author | Protocol title/ Publication title | Publication citation |
|---|---|---|
| Motzer R, Escudier B, Oudard S, et al. | Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial | Lancet 2008; 372(9637):449-56 |
The submission also presented unpublished updated data from a later cut-off (28 February
2008) on progression free survival and overall survival.
8. Results of Trials
The results for the primary outcome, progression-free survival (PFS), are shown below.
Progression-free survival (by independent review) as of 15 October 2007
| Analysis | Result (95% CI) | Level of statistical significance needed | |
|---|---|---|---|
| Progression-free survival – 191 events Everolimus (n=272): 101 (37%) events – 85 progressions, 16 deaths Placebo (n=138): 90 (65%) events – 82 progressions, 8 deaths | |||
| Comparison of time-to-first event curves | 2-sided stratified log rank test | P<0.0001 | P=0.0057 |
| Median progression-free survival Everolimus = 4.0 mo (3.7, 5.5) Placebo = 1.9 mo (1.8, 1.9) | |||
| CPH | HR = 0.30 (0.22, 0.40) | NA | |
NA: not applicable, CPH: Cox Proportional Hazards.
Kaplan-Meier curve of progression-free survival
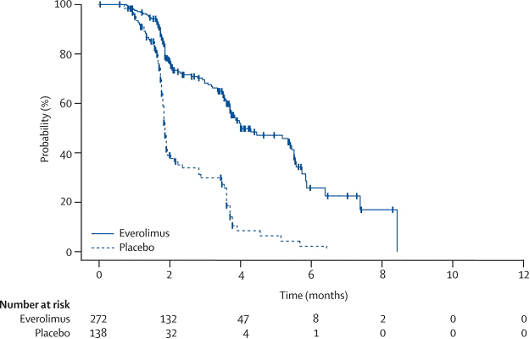
The PBAC noted that the RECORD-1 trial showed a statistically significant benefit
in PFS for everolimus compared with placebo although the magnitude of the gain was
small. The hazard ratios and 95% CIs were similar when progression was determined
by the investigator and when determined across various pre-planned subgroups (prior
treatment, age, gender and geographic region).
At the time of the 15 October 2007 analysis, median overall survival had not been
reached for the everolimus group and was 8.8 months for the placebo group.
The PBAC noted that there was no statistically significant difference between everolimus
and placebo in overall survival from the published data (HR=0.83, 95% CI: 0.50, 1.37,
p=0.23) or from the data included from the later cut-off.
The PBAC considered that the results for the secondary outcomes of Karnofsky performance
status, physical function, and QOL (quality of life) scores showed no statistically
significant differences in QOL and performance status between everolimus and placebo
treated patients. However, these results are difficult to interpret because of the
substantial crossover of placebo patients to everolimus treatment.
Everolimus has significant on-treatment toxicity compared to placebo, including increased
risk of serious infection, non-infectious pneumonitis, dyspnea, stomatitis, hyperglycaemia,
anaemia, lymphopenia as well as neurotoxicity.
For PBAC’s comments on these results, see Recommendation and Reasons.
9. Clinical Claim
The submission described everolimus as being superior in terms of
comparative effectiveness in PFS and inferior in terms of
comparative safety over placebo.
The PBAC considered that, based on the supporting data, the claim
of superior effectiveness is reasonable for progression-free
survival (PFS) but the difference in median PFS is small. However,
no benefit was demonstrated in overall survival or
quality-of-life.
10. Economic Analysis
The submission presented a stepped economic evaluation using a
decision-analytic Markov model comparing everolimus and placebo. It
consisted of three health states, stable disease, progressive
disease, and death, and had a 3-year horizon.
The model was most sensitive to assumptions regarding mortality
with uncertainties arising because of the wide confidence interval
around the survival estimate derived from the inverse censoring
probability weighted (ICPW) model and differences in risk of death
after progression in assumed differences in risks of death from
non-progressed and progressed disease.
The PBAC noted that using the survival hazard ratio from the trial,
the incremental cost per QALY gained increased the base case from
being in the range of $45,000 – $75,000 to in the range of
$75,000 – 105,000. Using the upper 95% confidence limit from
the submission’s IPCW analysis further increased the
incremental cost per QALY gained to be in the range of $105,000
– $200,000.
For PBAC’s comments, see Recommendation and
Reasons.
11. Estimated PBS Usage and Financial Implications
The financial cost per year to the PBS was estimated to be in the
range or $10 – $30 million per year in Year 5.
12. Recommendation and Reasons
The PBAC noted that when sorafenib is used as a first line agent,
sorafenib failed to show benefit in terms of progression free
survival (PFS) or overall survival but when used as a second-line
agent after failure of a cytokine showed a PFS of about 2.7 months.
Therefore, the study population for everolimus was a combination of
patients who had failed tyrosine kinase inhibitors (TKIs)
(sunitinib) and patients who had never been exposed to an effective
TKI (sorafenib). Given that sorafenib has failed to show any
benefit as a first line agent in RCC, the PBAC considered that this
may have influenced the benefit observed for everolimus.
The PBAC considered that there is a clinical need for options for
people who have failed sunitinib. However, sorafenib is not
available in Australia and therefore the requested PBS listing is
not entirely consistent with the population who will use the drug.
The PBAC considered that any future restriction should specify use
after disease progression on sunitinib rather than failure of
treatment as this implied resistance to treatment and there was no
way of measuring this as yet. In addition, the restriction should
also specify that everolimus should not be used after failure of
temsirolimus, both being mTOR inhibitors.
The PBAC noted that the RECORD-1 trial showed a statistically
significant benefit in PFS for everolimus compared with placebo
although the magnitude of the gain was small. The PBAC noted that
there was no statistically significant difference between
everolimus and placebo in overall survival. The PBAC considered
that the results indicated that radiological progression is a poor
predictor of survival in this context, particularly since the
difference was small. If disease free survival were a good marker
of overall survival then there should be some survival signal,
despite crossover to everolimus, but the benefit is small. By
comparing disease free survival and survival curves it appears that
factors other than disease free survival may be important for
predicting overall survival.
The PBAC noted that everolimus is also associated with serious
on-treatment toxicity such as non-infectious pneumonitis, dyspnea,
stomatitis, hyperglycaemia, anaemia, and lymphopenia. However, only
serious adverse events occurring at a rate of at least 2%, dyspnoea
and pneumonitis, were included in the model. The PBAC considered
that the results for Karnofsky performance status, physical
function, and QOL (quality of life) scores showed no statistically
significant differences in QOL and performance status between
everolimus and placebo treated patients. However, these results are
difficult to interpret because of the substantial crossover of
placebo patients to everolimus treatment.
Therefore, based on the supporting data, the claim of superior
effectiveness is reasonable for progression-free survival (PFS) but
the difference in median PFS is small. However, no benefit was
demonstrated in overall survival or quality-of-life.
The PBAC considered that the main area of uncertainty was in the
estimation of survival within study with inverse censoring
probability weighted (ICPW) method relative to conventional
methods. The PBAC noted that the ICPW technique attempts to adjust
for extreme confounding with crossover by re-weighting individuals
in the placebo arm. Individuals with low probability of crossover
are weighted almost the same as patients in the treatment arm,
while patients with high probability of crossover are weighted
close to 0. In addition the ICPW method has been classed in the
literature as ‘causal’ or ‘counterfactual’
in using group evidence to predicting what would have happened for
individuals who were censored.
The PBAC noted that the ICPW model generated a hazard ratio (HR)
estimate which continued to be applied in the model from 12 to 36
months. The PBAC considered that there is an overestimation in
benefits with extrapolation of treatment effects for progression
and survival beyond 12 months to 3 years and that the HR was
uncertain.
The PBAC agreed that there is an overall lack of supporting
evidence for extrapolation of within study estimated incremental
difference in median time of progression free survival to a
modelled result over 3 years.
The PBAC noted that using the survival hazard ratio from the trial,
the incremental cost per QALY gained increased the base case from
being in the range of $45,000 – $75,000 to in the range of
$75,000 – 105,000 . Using the upper 95% confidence limit from
the submission’s IPCW analysis further increased the
incremental cost per QALY gained to be in the range of $105,000
– $200,000.
The PBAC considered that although there was a clinical need for
everolimus and trial-based evidence of improved PFS, the magnitude
of that clinical benefit is highly uncertain in terms of overall
survival. The PBAC therefore rejected the submission on the basis
of uncertain clinical benefit and a high and uncertain
cost-effectiveness ratio.
Recommendation:
Reject
13. Context for Decision
The PBAC helps decide whether and, if so, how medicines should be
subsidised in Australia. It considers submissions in this context.
A PBAC decision not to recommend listing or not to recommend
changing a listing does not represent a final PBAC view about the
merits of the medicine. A company can resubmit to the PBAC or seek
independent review of the PBAC decision.
14. Sponsor’s Comment
The Sponsors will continue to work with PBAC to resolve the issues identified in this submission.




