Colistimethate sodium, powder for nebuliser solution, 1 million IU (equivalent to 80 mg colistimethate sodium), Tadim® - March 2011
Page last updated: 05 July 2011
Public Summary Document
Product: Colistimethate sodium, powder for
nebuliser solution, 1 million IU (equivalent to 80 mg
colistimethate sodium), Tadim®
Sponsor: Phebra Pty Ltd
Date of PBAC Consideration: March 2011
1. Purpose of Application
The submission sought dual Section 100 (Highly Specialised Drugs
Program) and Section 85 Authority Required listings for the
treatment of colonisation and infections of the lung due to
susceptible Pseudomonas aeruginosa in patients with cystic
fibrosis (CF).
Highly Specialised Drugs are medicines for the treatment of chronic
conditions, which, because of their clinical use or other special
features, are restricted to supply to public and private hospitals
having access to appropriate specialist facilities.
2. Background
This drug had not previously been considered by the PBAC.
3. Registration Status
Colistimethate sodium was TGA registered on 2 February 2011 for the
treatment of colonisation and infections of the lung due to
susceptible Pseudomonas aeruginosa in patients with cystic
fibrosis.
4. Listing Requested and PBAC’s View
Authority Required
Treatment of colonisation and infections of the lung due to
susceptible Pseudomonas aeruginosa in patients with cystic
fibrosis.
Section 100 (Highly Specialised Drugs Program)
Public hospital Authority Required (STREAMLINED)
Private hospital Authority Required
Treatment of colonisation and infections of the lung due to
susceptible Pseudomonas aeruginosa in patients with cystic
fibrosis.
For PBAC’s view, see Recommendation and
Reasons.
5. Clinical Place for the Proposed Therapy
Cystic fibrosis (CF) is a genetic disorder affecting cells in the
exocrine glands. This results in abnormal ion transport and
hydration at epithelial cell surfaces. In the lung, mucous
clearance is compromised, and this allows bacterial infections to
persist in static mucous. Over time, the resultant sub-optimal
inflammatory response to the infection contributes to lung damage
and progressive impairment of lung function. Cystic fibrosis
patients therefore have a greater risk of chronic lung infections
and intermittently receive courses of antibiotics by injection or
nebulisation to manage these infections. If chronically colonised
with the organism Pseudomonas aeruginosa they may require
multiple courses of intravenous antibiotics for the management of
pulmonary exacerbations. The number of CF patients in Australia in
2008 as recorded by the Australian Cystic Fibrosis Data Registry
(ACFDR) was 2,843.
The submission proposed that the place in therapy of colistimethate
sodium powder for nebuliser solution is as a short term treatment
for the eradication of Pseudomonas aeruginosa and as long
term therapy alternating with tobramycin to control chronic
Pseudomonas aeruginosa infections.
6. Comparator
The submission nominated no treatment (or placebo) as the main
comparator. The submission also nominated inhaled tobramycin as an
alternative comparator.
The PBAC agreed that placebo (or no treatment) given in addition to
standard care (other than nebulised anti-pseudomonal antibiotics)
was an acceptable comparator.
For PBAC’s view, see Recommendation and
Reasons.
7. Clinical Trials
The submission presented the following studies in support of the comparative effectiveness
of colistimethate relative to no treatment/placebo or tobramycin for the management
of P. aeruginosa infection in patients with CF:
1. Early/intermittent P. aeruginosa infection:
- One direct randomised trial comparing colistimethate + ciprofloxacin with no treatment (Valerius 1991), in addition to standard care other than anti-pseudomonal treatment. Standard care included antimicrobial therapy against bacterial pathogens other than P. aeruginosa, was continued in both groups. Patients in the colistimethate + ciprofloxacin arm were treated for 3 weeks at entry to the trial and each time P. aeruginosa was isolated from subsequent monthly sputum samples. The mean number of treatment courses was not reported in the published paper.
- One direct randomised trial comparing colistimethate + ciprofloxacin with tobramycin (Proesmans 2009); and
- Three supplementary studies: one historical control study comparing the treatment of P. aeruginosa infection before and after the use of an intensive colistimethate/ciprofloxacin protocol (Frederiksen 1997), and two prospective case series (Brochet 2007 and Giugno 2010).
2. Chronic P. aeruginosa infection:
- Two direct randomised trials comparing colistimethate with placebo, in addition to standard care other than anti-pseudomonal treatment (Jensen 1987 and Day 1988);
- One direct randomised trial comparing colistimethate with tobramycin (Hodson 2002); and
- One comparative study comparing colistimethate with tobramycin (Nikonova 2010).
No studies were identified by the submission which investigated colistimethate for
the treatment of acute exacerbations of lung disease in patients with CF and with
P. aeruginosa infection.
The table below details the published trials presented in the submission:
| Trial ID/ First author | Protocol title / Publication title | Publication citation |
| Early/intermittent Pseudomonas aeruginosa infection | ||
| Colistimethate vs no treatment RCT | ||
| Valerius NH et al | Prevention of chronic Pseudomonas aeruginosa colonisation in cystic fibrosis by early treatment. | Lancet. 1991; 338(8769): 725-6 |
| Valerius NH et al | Prevention of Chronic colonization with Pseudomonas aeruginosa in patients with CF by early treatment with ciprofloxacin and colistin aerosol inhalations. | Conference Proceedings. 4th North American Cystic Fibrosis Conference 1990 |
| Colistimethate vs tobramycin RCT | ||
| Proesmans M et al | Eradication of recent Pseudomonas aeruginosa isolation: TOBI versus colistin/ciprofloxacin [conference abstract] | North American Cystic Fibrosis Conference; 2009 Oct 15-17; Minneapolis, Minnesota. Pediatric Pulmonology. 2009; 44(Suppl 32): 321 |
| Proesmans M et al | Eradication of recent Pseudomonas aeruginosa isolation: TOBI versus colistin/ ciprofloxacin [conference abstract]. | The 31st European Cystic Fibrosis Society Conference; 2008 June 11-14; Prague, Czech Republic. Journal of Cystic Fibrosis. 2008; 7(Suppl 2):S64 |
| With vs without intensive colistimethate/ciprofloxacin protocol study a | ||
| Frederiksen B et al | Antibiotic treatment of initial colonization with Pseudomonas aeruginosa postpones chronic infection and prevents deterioration of pulmonary function in cystic fibrosis. | Pediatric Pulmonology. 1997; 23(5): 330-5 |
| Frederiksen B et al | Delay of recurrence of Pseudomonas aeruginosa in patients with cystic fibrosis with inhaled colistin and oral ciproxin: a comparison between 3 weeks and 3 months of treatment [conference abstract]. | 11th Annual North American Cystic Fibrosis Conference; 1997 Oct. 23-26; Nashville, Tennessee. Pediatric Pulmonology. 1997; Suppl 14: 288 |
| Prospective case series | ||
| Brochet MS et al | Comparative efficacy of two doses of nebulized colistimethate in the eradication of Pseudomonas aeruginosa in children with cystic fibrosis. | Canadian Respiratory Journal. 2007; 14(8): 473-9 |
| Giugno H et al | Early antibiotic treatment for eradication of initial infection by Pseudomonas aeruginosa in patients with cystic fibrosis. [Spanish] | Archivos Argentinos de Pediatria. 2010; 108(2): 141-7 |
| Chronic Pseudomonas aeruginosa infection | ||
| Colistimethate vs placebo RCTs | ||
| Jensen T et al | Colistin inhalation therapy in cystic fibrosis patients with chronic Pseudomonas aeruginosa lung infection. | Journal of Antimicrobial Chemotherapy. 1987; 19(6): 831-8 |
| Day AJ et al | Evaluation of inhaled colomycin in children with cystic fibrosis [conference abstract] | 10th International Cystic Fibrosis Congress. Sydney Australia R(c)3p. 1988. |
| Colistimethate vs tobramycin RCT | ||
| Hodson ME et al | A randomised clinical trial of nebulised tobramycin or colistin in cystic fibrosis. | European Respiratory Journal. 2002; 20(3): 658-64 |
| Hodson ME et al. | New clinical evidence from the European tobramycin trial in cystic fibrosis. | Journal of Cystic Fibrosis. 2002; 1(Suppl 2): 199-202 |
| Govan JR et al. | Insights into cystic fibrosis microbiology from the European tobramycin trial in cystic fibrosis. | Journal of Cystic Fibrosis. 2002; 1(Suppl 2): 203-8 |
| Adeboyeku D et al | Open follow-up study of tobramycin nebuliser solution and colistin in patients with cystic fibrosis. | Journal of Cystic Fibrosis. 2006; 5(4):261-3 |
| Hodson ME et al | Randomised UK/Eire clinical trial of the efficacy and safety of tobramycin 300mg/5ml nebuliser solution for nebulised colistin [conference abstract]. | 14th Annual North American Cystic Fibrosis Conference, Baltimore, Maryland, Nov 9-12, 2000. Pediatric Pulmonology. 2000; Suppl 20: 248-249 |
| Colistimethate vs tobramycin study b | ||
| Nikonova VS et al | Efficacy and safety of tobramycin and Colistin for inhalation in children with cystic fibrosis from Moscow region. [conference abstract] | 33rd European Cystic Fibrosis Conference Valencia, Spain 16-19 June 2010 Journal of Cystic Fibrosis. 2010; 9: S54 |
RCT = randomised controlled trial
a Frederiksen 1997 was a historical control study, not a single-arm study, as suggested
in the submission.
b Nikonova 2010 is called a comparative study, rather than a RCT, as in the Commentary,
as the conference abstract of this study did not specify whether patients were randomised
to the different treatment arms.
8. Results of Trials
Early/intermittent P. aeruginosa infection
Four of the five studies included in the submission reported the effectiveness of
colistimethate in addition to ciprofloxacin for the treatment of early/intermittent
P. aeruginosa infection; only the study Brochet 2007 reported the effectiveness of colistimethate
as a single-agent therapy.
The primary outcomes in the submission included the eradication of P. aeruginosa and prevention or delay of chronic P. aeruginosa infection.
a) Colistimethate + ciprofloxacin versus no treatment (Valerius 1991)
The proportion of patients free of chronic Pseudomonas aeruginosa infection reported in Valerius 1991 is shown below:
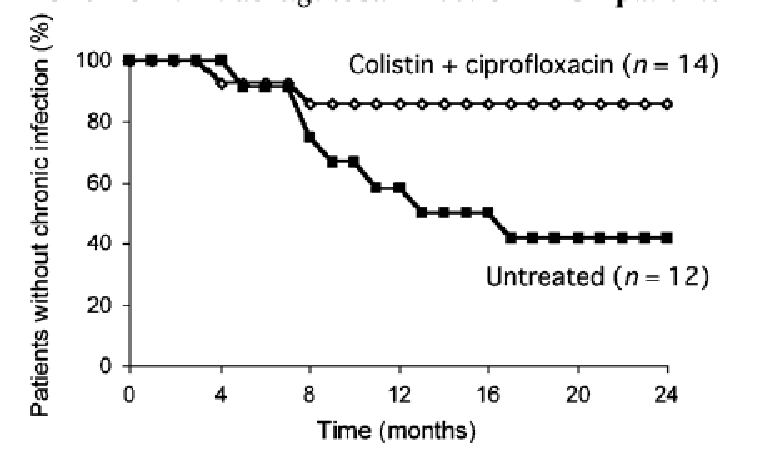
The proportion of patients developing chronic Pseudomonas aeruginosa infection reported in Valerius 1991 is shown in the table below:
| Outcome | Colistimethate + ciprofloxacin | No treatment | Treatment effect a RR [95% CI] |
|---|---|---|---|
| Number of patients developing chronic Pseudomonas aeruginosa infection during 27 months b | 2/14 | 7/12 | |
| Proportion | 14.3% | 58.5% | 0.25 [0.06, 0.96] |
CI = confidence interval; RR = relative risk
a Colistimethate + ciprofloxacin vs no treatment
b The mean follow-up time for those who did not develop chronic P. aeruginosa infection was 17.4 months.
For PBAC’s comments on these results, see Recommendation and Reasons.
b) Colistimethate + ciprofloxacin versus tobramycin (Proesmans 2009)
The results of Pseudomonas aeruginosa eradication reported in Proesmans 2009 are shown below:
| Outcome | Colistimethate + ciprofloxacin | Tobramycin | Treatment effect a RR [95% CI] |
|---|---|---|---|
| Proportion of patients with eradication of Pseudomonas aeruginosa at end of treatment | 14/15 (93.3%) | 14/17 (82.4%) | 1.13 [0.88, 1.47] |
| Proportion of patients with eradication of Pseudomonas aeruginosa at 6 months follow-up | 7/15 (46.7%) | 7/17 (41.2%) | 1.13 [0.52, 2.48] |
CI = confidence interval; RR = relative risk
a colistimethate + ciprofloxacin vs no treatment
Proesmans 2009 is an ongoing trial, which recruited 50 patients. The results presented
in the submission related to an interim analysis conducted on 32 patients. The proportion
of patients with eradication of P. aeruginosa was higher in the colistimethate + ciprofloxacin arm than in the tobramycin-treated
arm both at the end of 3 months of treatment (93.3% vs 82.4%) and at 6 months follow-up
(46.7% vs 41.2%).
For PBAC’s comments on these results, see Recommendation and Reasons.
c) Supportive studies (Frederiksen 1997, Brochet 2007 and Giugno 2010)
In Frederiksen 1997, only 16% of the 48 patients treated with three-step colistimethate/ciprofloxacin
(according to treatment protocol) developed chronic P. aeruginosa infection during 3.5 years, compared with the rate of 72% in historical controls
(43 patients with P. aeruginosa cultured in the period between November 1983 and June 1987).
The PBAC noted that there was a trend suggesting that 3 months of high-dose treatment
with inhaled colistimethate (2 million international unit (IU), three times a day
(tid)) in addition to oral ciprofloxacin produced the best results with respect to
postponement or prevention of chronic P. aeruginosa infection.
Results from the prospective case series, Brochet 2007 and Giugno 2010, supported
the evidence from the direct randomised trials by demonstrating beneficial effects
from colistimethate ± ciprofloxacin with respect to eradication of P. aeruginosa in CF patients with early/intermittent P. aeruginosa infection. The lack of a comparator, however, hindered quantification of the magnitude
of the effect.
Chronic P. aeruginosa infection
None of the four studies (Jensen (1987), Nikonova (2010), Day 1988 and Hodson 2002)
investigated the impact of alternating inhaled tobramycin and colistimethate on a
1-month on/off cycle, which is the most commonly used regimen recommended by the Respiratory
Therapeutic Guidelines. The durations of treatment in these studies (4 weeks – 6 months)
are relatively short, whereas various clinical guidelines and PI documents recommend
long-term therapy for patients with chronic P. aeruginosa infection.
Changes in lung function (FEV1 and forced vital capacity (FVC)) were nominated in the submission as the primary
outcome.
Nikonova (2010) included CF patients with both early P. aeruginosa infection (colistimethate arm: 6/9; tobramycin arm: 2/32) and chronic infection.
It was also possible that Day 1988 and Hodson 2002 recruited a mixture of early/chronic
infection, since their selection criteria, provided in the submission, did not specify
stage of P. aeruginosa infection.
a) Colistimethate versus placebo (Jensen 1987 and Day 1988)
Absolute changes in lung function reported in Jensen 1987
|
Treatment day |
Colistimethate (n=18) |
Placebo |
Mean difference |
| Mean FEV1 % predicted ± SD | |||
|
Day 0 |
71 ± 25 |
79 ± 29 |
|
|
Day 90 |
60 ± 24 |
62 ± 29 |
|
|
Changeb (Day 90 – Day 0) |
-11 ± 6c |
-17 ± 11c |
6.0 [-1.1, 13.1] |
| Mean FVC % predicted ± SD | |||
|
Day 0 |
86 ± 26 |
89 ± 31 |
|
|
Day 90 |
79 ± 25 |
71 ± 28 |
|
|
Changed (Day 90 – Day 0) |
-7 ± 8e |
-18 ± 14d |
11.0 [1.9, 20.1] |
CI = confidence interval; FEV1 = forced expiratory volume in one second; FVC = forced
vital capacity; SD = standard deviation
a Colistimethate – placebo.
b A decrease in FEV1 % suggests a deterioration of lung function.
c Non-statistically significant difference for colistimethate versus placebo group
(p >0.05)
d A decrease in FVC% suggests a deterioration of lung function.
e Statistically significant difference for colistimethate versus placebo group (p <0.01)
In the treatment of chronic Ps. aeruginosa, the Jensen 1987 trial demonstrated a decrease in FEV1 % predicted in both colistimethate and placebo arms with the difference between treatments
not reaching statistical significance.
Day 1988 was a crossover trial, which compared 6-month inhaled colistimethate treatment
with inhaled placebo in 14 patients. Both FVC % predicted and FEV1 predicted decreased in the placebo arm, but were maintained in colistimethate-treated
patients. Six-month treatment with colistimethate was associated with a significantly
greater mean FVC % predicted when compared with placebo (74% vs 67.5%, p<0.05).
For PBAC’s comments on these results, see Recommendation and Reasons.
b) Colistimethate versus tobramycin (Hodson 2002 and Nikonova 2010)
In trial Hodson 2002, tobramycin was associated with a greater improvement in FEV1 % predicted at Week 4, when compared with colistimethate (relative change; 6.70%
vs 0.37%); the difference was statistically significant, based on the p-value of 0.008
reported in the published paper (using Wilcoxon rank-sum test). The PBAC noted that
tobramycin showed a statistically greater improvement in the relative change in mean
FEV1 % predicted than colistimethate. However, the PBAC had concerns about the lack of
data on absolute change in FEV1 % predicted, the open label study design, the differential background exposure of
study drug between patients in the treatment arms and the insufficient time allowed
for follow-up.
In the trial Nikonova 2010, data were sourced from a conference abstract. Eradication
rates were higher in colistimethate-treated patients, no matter whether the P. aeruginosa infection was early or chronic, when compared with patients in the tobramycin arm
(early infection: 67% vs 50%; chronic infection: 67% vs 43%). The reporting of P. aeruginosa eradication in patients with chronic infection was unexpected and required further
investigation.
For PBAC’s comments on these results, see Recommendation and Reasons.
The PBAC noted that across included studies, about 10% – 70% of the colistimethate-treated
patients developed at least one adverse event. Serious adverse events occurred in
less than 12% of patients. Cough was the most commonly reported adverse event associated
with nebulised colistimethate treatment, with a rate of between 18% and 47%. However,
this adverse event was also observed in patients treated with inhaled tobramycin (9%)
and inhaled placebo (>5%).
9. Clinical Claim
The submission claimed colistimethate as superior in terms of comparative effectiveness
and inferior in terms of comparative safety over the nominated comparator, no treatment,
when used for the treatment of either early/intermittent or chronic P. aeruginosa infection in patients with CF.
For PBAC’s view, see Recommendation and Reasons.
10. Economic Analysis
1. Treatment of early/intermittent P. aeruginosa infection
The submission presented a trial-based economic evaluation based on Valerius 1991.
The trial-based economic evaluation assessed the cost-effectiveness of a three month
regimen of nebulised colistimethate and oral ciprofloxacin, relative to no treatment.
The time horizon is 27 months.
The resources considered in the economic evaluation were the pharmaceutical costs
for colistimethate and ciprofloxacin, and the cost of diluent to reconstitute colistimethate
for nebulisation.
The health outcome used in the evaluation was the proportion of patients free of
chronic P. aeruginosa infection at 27 months.
The submission estimated the incremental cost per additional patient free of P.aeruginosa infection at 27 months was less than $15,000.
The submission also presented a cost-minimisation analysis for colistimethate versus
inhaled injectable tobramycin in the treatment of early/intermittent P.aeruginosa infection. The submission assumed that both nebulised drugs would be given concomitantly
with oral ciprofloxacin, however, the only clinical evidence presented was Proesmans
2009 in which the tobramycin arm was given as monotherapy over 28 days. The comparative
effectiveness of colistimethate and tobramycin when both are administered concomitantly
with ciprofloxacin for three months was not presented.
The submission assumed that patients will require two 500 mg/5 mL vials of injectable
tobramycin per day in order to administer the recommended dose of 300 mg twice daily.
The submission did not present any trial evidence to justify the assumed equi-effective
doses of colistimethate and nebulised tobramycin when both are administered concomitantly
with ciprofloxacin for three months, therefore the validity of this analysis was highly
uncertain.
2. Management of chronic infection
The submission presented a trial-based economic evaluation based on Jensen 1987. The
submission presented the cost-effectiveness of a 90-day regimen of nebulised colistimethate
compared to placebo over a time horizon of 90 days.
The resources considered in the economic evaluation were the pharmaceutical costs
for colistimethate and the cost of diluent. The submission did not include costs associated
with management of acute exacerbations, such as hospitalisations and administration
of IV antibiotics. The health outcome is the absolute change in FEV1 % predicted from baseline to day 90.
The incremental cost per additional 1% FEV1 % predicted at day 90 was estimated to be less than $15,000.
For PBAC’s comments, see Recommendation and Reasons.
11. Estimated PBS Usage and Financial Implications
The likely number of patients per year was estimated by the submission to be less
than 10,000 in Year 4. The submission’s estimate was considered uncertain.
The financial cost per year to the PBS was estimated by the submission to be less
than $10 million in Year 4. The submission’s estimate was considered uncertain.
12. Recommendation and Reasons
The PBAC considered that the poor quality of the clinical data presented in the submission did not provide a basis for listing. The clinical trials which formed the basis of the submission were sub-optimal in design, with issues such as low patient numbers, possible confounding of results from prior use of colistimethate, and concomitant ciprofloxacin use in the treatment arm without ciprofloxacin in the control arm. At the hearing, the spokesperson emphasised that the primary focus was decreasing the loss of lung function and that better long-term outcomes resulted from earlier starts on therapy.
The PBAC agreed that placebo (or no treatment) given in addition to standard care
(other than nebulised anti-pseudomonal antibiotics) was an acceptable comparator.
However, it was noted that for treatment of early/intermittent Ps. aeruginosa infection current clinical guidelines recommended that both colistimethate and nebulised
tobramycin should be administered in addition to oral and/or IV antibiotics. The submission
did not provide sufficient data for the comparison of interest which is cycles of
tobramycin for one month, placebo for one month vs tobramycin for one month, colistimethate
for one month.
In the treatment of early/intermittent Ps. aeruginosa infection, the Valerius 1991 trial found that a significantly lower rate of patients
treated with colistimethate and ciprofloxacin became chronically colonised with Ps. aeruginosa (14.3%) than untreated patients (58%). However, the PBAC considered the results should
be interpreted with caution, given the small patient numbers, potential inadequacies
in the measures taken to minimise bias, the differential number of patients with recent
Ps. aeruginosa isolates between the intervention arms (4 out of 14 patients in the colistimethate
+ ciprofloxacin group vs 1 out of 12 patients in the no treatment group), the possible
differential duration of follow-up between the patients who did not develop Ps. aeruginosa infection and those that did and the changing standards of care between then and
now. Similar issues were evident with the other key randomised trial (Proesman 2009),
an interim analysis on 32 patients, which showed there was no statistically significant
difference between colistimethate plus ciprofloxacin versus tobramycin in eradication
of Ps. aeruginosa.
In the treatment of chronic Ps. aeruginosa, the Jensen 1987 trial demonstrated a decrease in FEV1 % predicted in both colistimethate
and placebo arms with the difference between treatments not reaching statistical significance.
The PBAC noted that a clinical score used in the study favoured colistimethate, but
the clinical importance of the observed changes in the clinical score was uncertain.
The validity of the other placebo-controlled trial (Day 1988) was limited by its crossover
study design, due to concerns about carryover and uncertainty about the clinical relevance
of the observed difference in FVC % predicted. For the trial against active comparator
(Hodson 2002), tobramycin showed a statistically greater improvement in the relative
change in mean FEV1 % predicted than colistimethate. However, the PBAC had concerns about the lack of
data on absolute change in FEV1 % predicted, the open label study design, the differential background exposure of
study drug between patients in the treatment arms and the insufficient time allowed
for follow-up.
The submission presented a trial-based economic evaluation based on Valerius 1991
that assessed the cost-effectiveness of a three month regimen of nebulised colistimethate
and oral ciprofloxacin relative to no treatment. The incremental cost per additional
patient free of Ps. aeruginosa infection at 27 months was less than $15,000. The PBAC considered that the outcome
was difficult to interpret given that the mean duration of follow-up for patients
who did not become chronically infected was only 17.4 months and the value of remaining
free of chronic Ps. aeruginosa infection over this duration of time (i.e. 17.4 months) in terms of health or utility
gain was not quantified in this outcome measure. In addition, the outcome was confounded
by the concurrent use of ciprofloxacin in the colistimethate treatment arm. The cost
minimisation analysis against tobramycin over a three month horizon assumed that both
nebulised drugs would be given concomitantly with oral ciprofloxacin, however the
only evidence presented was Proesmans 2009 in which the tobramycin arm was given as
monotherapy over 28 days.
For the management of chronic infection the PBAC noted that a trial-based economic
evaluation based on Jensen 1987 was used which presented the cost-effectiveness of
a 90 day regimen of nebulised colistimethate compared to placebo over a time horizon
of 90 days. The incremental cost per additional 1% FEV1 % predicted at Day 90 was less than $15,000. The PBAC noted that there was no statistically
significant difference in the absolute change in FEV1 % predicted in this trial and no evidence presented that any treatment effect was
maintained over a clinically relevant time. The PBAC considered that it was difficult
to interpret in terms of final patient-relevant outcomes, such as hospitalisations,
survival, and quality adjusted survival, and that the age of the trial made it unlikely
that the results were directly applicable to the population targeted by the PBS restriction.
The PBAC considered that use in the model of annual mortality rates of 10% (acute)
and 2% (chronic) based on data at least 30 years old was likely to be a substantial
over-estimate, as regular anti-pseudomonal therapy was now part of standard care in
patients with chronic Ps. aeruginosa infection.
The PBAC therefore rejected the submission on the basis of uncertain clinical benefit,
uncertain clinical place in therapy, and extrapolating from that, uncertain cost-effectiveness.
The PBAC noted the consumer comments received for this item and the advice of the
Highly Specialised Drugs Working Party that concluded colistimethate did not meet
all the criteria for listing as a highly specialised drug.
Recommendation:
Reject
13. Context for Decision
The PBAC helps decide whether and, if so, how medicines should be subsidised in Australia. It considers submissions in this context. A PBAC decision not to recommend listing or not to recommend changing a listing does not represent a final PBAC view about the merits of the medicine. A company can resubmit to the PBAC or seek independent review of the PBAC decision.
14. Sponsor’s Comment
The sponsor has no comment.




