Eribulin Mesilate, solution for injection, 1mg in 2mL Halaven® - March 2013
PDF printable version of this page
Public Summary Document
Product: Eribulin Mesilate, solution for injection, 1mg in 2mL Halaven®
Sponsor: Eisai Australia Pty Limited
Date of PBAC Consideration: March 2013
1. Purpose of Application
The submission requested Section 100 Efficient Funding of Chemotherapy Program Private Hospital / Private Clinic Authority required and Public Hospital Authority required (STREAMLINED) listings for the treatment of a patient with locally advanced or metastatic breast cancer who has progressed after at least two chemotherapeutic regimens for advanced disease. Prior therapy should include an anthracycline and a taxane unless these treatments were unsuitable for the patient.
2. Background
This product had not been considered by the PBAC previously.
3. Registration Status
Eribulin was registered by the TGA on 4 September 2012 as follows:
- Eribulin monotherapy is indicated for the treatment of patients with locally advanced or metastatic breast cancer who have progressed after at least two chemotherapeutic regimens, for advanced disease. Prior therapy should have included an anthracycline and a taxane, unless patients were not suitable for these treatments.
|
4. Listing Requested and PBAC’s View Section 100 Efficient Funding of Chemotherapy Program Private Hospital / Private Clinic Authority required Public Hospital Authority required (STREAMLINED) For the treatment of patients with locally advanced or metastatic breast cancer who have progressed after at least two chemotherapeutic regimens for advanced disease which includes capecitabine if indicated. Prior therapy should have included an anthracycline and a taxane unless patients were contraindicated for these treatments. |
The PBAC noted that in the sponsor’s pre-sub-committee response, a revised restriction was proposed where it was specified that use of eribulin should follow capecitabine if indicated (shown in italics above). The PBAC noted that the TGA approved indication for eribulin did not specify use after capecitabine.
For PBAC’s view, see Recommendation & Reasons.
5. Clinical Place for the Proposed Therapy
The submission’s proposed clinical management algorithm for the intended use of eribulin is presented below.
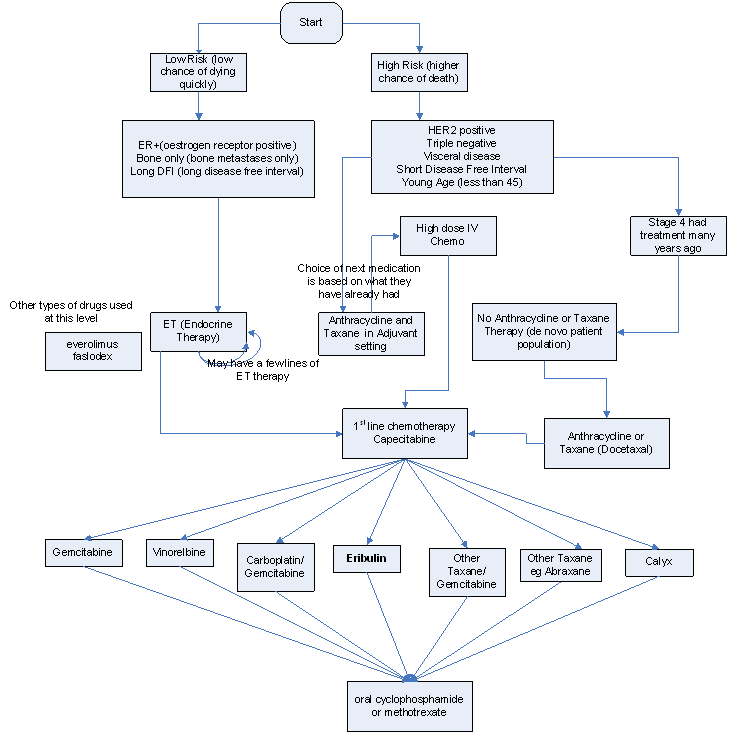
The PBAC noted that the proposed clinical algorithm suggested that a patient with low risk advanced breast cancer can access eribulin without prior anthracycline and taxane treatment, which contradicted the proposed PBS restriction. Moreover, the proposed clinical algorithm assumed every patient would have received capecitabine prior to the treatment with eribulin.
The PBAC considered that the proposed place of therapy after the use of capecitabine, was clinically inappropriate. The PBAC considered that the appropriate place of therapy was likely to be after anthracyclines and taxines but that eribulin may be used before or after capecitabine. The PBAC also noted that in HER2 positive breast cancer, clinicians may want to use eribulin in combination with trastuzumab.
6. Comparator
The submission nominated treatment of Physician’s Choice (TPC), the alternative treatment the investigator elects as appropriate; defined as any single agent chemotherapy, hormonal therapy or targeted therapy approved for the treatment of cancer (for any tumor type – not just breast cancer), or best supportive care or radiotherapy, administered according to local practice, if applicable.
For PBAC’s view, see Recommendation and Reasons.
7. Clinical Trials
The submission presented a multi-centre, open-label, phase III randomised trial (EMBRACE) comparing eribulin (508 patients) or TPC (254 patients). Randomised patients were stratified by geographical region, previous capecitabine treatment and human epidermal growth factor receptor 2 status.
The submission also presented two phase II trials (E389-A001-201 and E7389-G000-211) as supporting evidence. Trial E389-A001-201 was an open label single arm multi-centre study (N=104). The dosing regimen changed during the study and the submission focussed on the 33 patients receiving eribulin 1.4mg/m2 day 1 and 8 of 21 day cycle (TGA approved indication). Study E7389-G000-211 was an open label single arm multi-centre study in patients who had between two and five prior treatments (including an anthracycline, taxane and capecitabine).
The table below details the published trials presented in the submission.
|
Trial ID/ First author |
Protocol title/ Publication title |
Publication citation |
|---|---|---|
|
Direct randomised trial EMBRACE Cortes J, et al |
Eribulin Monotherapy Versus Treatment of Physician’s Choice in Patients with Metastatic Breast Cancer (EMBRACE): A Phase 3 Open-Label Randomised Study. |
The Lancet 2011; 377(9769):914-923 |
|
Supplementary randomised trials E7389-A001-201 Vahdat LT, et al.
|
Phase II Study of Eribulin Mesylate, A Halichondrin B Analog, in Patients with Metastatic Breast Cancer Previously Treated with an Anthracycline and a Taxane. |
Journal of Clinical Oncology 2009;27(18):2954-2961.
|
|
E7389-G000-211 Cortes J, et al.
|
Phase II Study of the Halichondrin B Analog Eribulin Mesylate in Patients with Locally Advanced or Metastatic Breast Cancer Previously Treated with an Anthracycline, a Taxane, and Capecitabine. |
Journal of Clinical Oncology 2010;28(25):3922-3928.) |
8. Results of Trials
The primary efficacy endpoint in the EMBRACE trial was overall survival (OS). Results are presented in the table below.
The submission presented the overall survival (OS) as the primary endpoint for the EMBRACE trial.
Results of Overall Survival for EMBRACE Trial (Primary analysis)
|
|
Treatment arm Eribulin (N=508) |
TPC (N=254) |
||
|---|---|---|---|---|
| Overall Survival, days | ||||
| Stratified log-rank test | ||||
|
Hazard ratio matching the primary analysis (eribulin/TPC)a |
||||
|
Hazard ratio (eribulin/TPC): Sensitivity analysis also adjusted for number of prior chemotherapy regimens and ER statusb |
||||
|
Number of patients who died, n (%) |
274 (53.9) |
148 (58.3) |
||
|
Number of patients who censored, n (%) |
234 (46.1) |
106 (41.7) |
||
|
Median (95% CI) |
399 (360, 434) |
324 (282, 380) |
||
|
3rd Quartile (95%) |
650 (573, NE) |
NE (547, NE) |
||
|
Difference in Medians (95% CI) |
75.0 (21.4, 128.6) |
|||
|
p-value |
0.041 |
|||
|
1-year survival rate (proportion) |
0.539 |
0.437 |
||
|
95% CI |
(0.492, 0.586) |
(0.371, 0.502) |
||
|
2-year survival rate (proportion) |
0.219 |
0.272 |
||
|
95% CI |
(0.148, 0.290) |
(0.188, 0.355) |
||
|
Estimate |
0.809 |
|||
|
95% CI |
(0.660, 0.991) |
|||
|
Estimate |
0.810 |
|||
|
95% CI |
(0.660, 0.994) |
|||
Abbreviations: CI = Confidence interval; ER = Estrogen receptor; HER/neu = human epidermal growth factor receptor 2; ITT = Intent-to-treat; NE = Not estimable due to insufficient events; TPC = Treatment of Physician’s Choice.
Note: a Hazard ratio based on a Cox model including HER2/neu status, prior capecitabine treatment, and geographical region as strata.
b Hazard ratio based on a Cox model including HER2/neu status, prior capecitabine treatment, geographical region as strata, and number of prior chemotherapy regimens, and estrogen receptor status as covariates.
Source: Table B.6.1 p 77 of the submission.
The PBAC noted in the primary analysis, OS was significantly increased with eribulin compared with TPC (p=0.041); the hazard ratio (HR) using a Cox model stratified by geographic region, prior capecitabine and HER2 receptor status was 0.809 (95% CI: 0.660, 0.991). Median OS was 399 days (95% CI: 360, 434) in the eribulin group and 324 days (95% CI: 282, 380) in the TPC group.
The submission also presented an updated analysis carried out at 77% of events, with the median survival of the eribulin group (median: 403 days/13.2 months) compared with the TPC group (median: 321 days/10.5 months). The results showed an improvement of 82 days/2.7 months in favour of eribulin compared with TPC (HR 0.805, 95% CI: 0.677, 0.958, nominal p=0.014).
The submission stated that eribulin has a manageable non-inferior safety profile, given the percentages of patients who experienced death or treatment related SARs were similar between eribulin and TPC patients, 25% vs. 25.9%, respectively.
The PBAC noted that the incidences of neutropenia, leukopenia and peripheral neuropathy were higher in the eribulin group (51.7%, 23.1% and 34.6% respectively) than in the TPC group (29.6%, 11.3%, and 16.2%). The PBAC also noted that the use of granulocyte stimulating factors to reduce the risk of febrile neutropenia was much higher in eribulin arm (17.7%) than TPC arm (7.7%).
The PBAC further noted the presentation of the differential adverse events given in the Sponsor’s hearing which compared adverse events with eribulin to other chemotherapy products.
For PBAC’s view, see Recommendation & Reasons.
9. Clinical Claim
The submission claimed eribulin to be superior in terms of comparative effectiveness and ‘manageably’ non-inferior in terms of comparative safety over the TPC.
For PBAC’s view, see Recommendation & Reasons.
10. Economic Analysis
The submission presents a trial-based economic evaluation, based on the key direct randomised trial (EMBRACE) and implementing a modelled evaluation based on extrapolated survival curves. The PBAC considered that, the availability of updated OS data in the PSCR rendered the extrapolation of the survival curves unnecessary. The types of economic evaluation presented were a cost-utility analysis and a cost-effectiveness analysis (with Life Years as the outcome). The cost-utility analysis is presented in the form of a Cost-Quality Adjusted Time Without Symptoms or Toxicity (QTWiST) analysis. The PBAC considered that this approach was appropriate, however noted that the economic analysis did not adequately capture the disutility of treatment.
Using the updated survival data provided in the PSCR, the ICER for the intention to treat (ITT) population was between $45,000 and $75,000 per life year gained (LYG). The ICER for the post capecitabine subgroup was lower within the same range.
Vinorelbine was nominated as the comparator for the economic evaluation. Given the difficulty of identifying a single comparator, the PBAC considered the pragmatic selection of vinorelbine as the comparator in the economic analysis was reasonable.
For PBAC’s view, see Recommendation & Reasons.
11. Estimated PBS Usage and Financial Implications
The submission estimated the total net cost to the PBS to be between $10 – $30 million over the first 5 years of listing (excluding co-payments).
The PBAC considered there were a number of issues concerning the submission’s estimates of the financial implications of listing eribulin, namely:
- The underestimate of the number of eligible patients;
- The uncertainty in the number of eligible patients who would decide (in consultation with their clinician) to be treated with eribulin;
- The costs for adverse events;
- The uncertainty of duration of treatment in the PBS population being consistent with that in the clinical trial;
- The underestimate of cost per infusion because of incorrect application of Efficient Funding of Chemotherapy (EFC) methodology.
12. Recommendation and Reasons
The PBAC rejected the submission on the basis of unacceptably high and uncertain cost-effectiveness and because of a lack of clarity regarding the clinical place of the product.
With regard to the submission’s nominated comparator, the PBAC noted that the available data do not provide any clarity about the relative mix of agents used in the Australian setting and that the EMBRACE study had taken a pragmatic approach of Treatment of Physician’s Choice (TPC). This was defined as any single agent chemotherapy, hormonal therapy or targeted therapy approved for the treatment of cancer (for any tumor type – not just breast cancer), or best supportive care or radiotherapy, administered according to local practice, if applicable.
In the EMBRACE trial, TPC consisted of chemotherapy (97%) and hormonal therapy (3%). Chemotherapy included vinorelbine, gemcitabine, capecitabine, taxanes, anthracyclines, cisplatin, carboplatin, cyclophosphamide, etoposide, mitomycin, fluorouracil and methotrexate. Hormonal therapy included fulvestrant, letrozole, exemestane and tamoxifen. All drugs are PBS listed except mitomycin and fulvestrant. There are a small number of recipients of these drugs in EMBRACE (n=1 for mitomycin and n= 4 for fulvestrant).
The PBAC considered that the mix of agents used in the economic model in the pre-subcommittee response may not accurately represent Australian clinical practice.
From the data presented on overall survival, the PBAC considered that eribulin had demonstrated superior efficacy over the comparator based on the overall survival results. However, the PBAC did not consider the claim of non-inferior toxicity was supported, based on additional G-CSF requirements.
The PBAC acknowledged that eribulin was an effective drug that offered a small survival benefit at the end of life. However, the PBAC considered that the clinical place of eribulin was not well defined. The PBAC considered that the requested restriction for treatment after the use of capecitabine was not clinically justified. The PBAC considered that the appropriate place of therapy was likely to be after anthracyclines and taxanes but that eribulin may be used before or after capecitabine. The PBAC also noted that in HER2 positive breast cancer, clinicians may want to use eribulin in combination with trastuzumab.
From the results of the economic analysis, the PBAC considered the ICER presented in the PSCR (verified by the Evaluators) based on the OS results observed in the updated data for the comparison versus TPC of between $45,000 and $75,000 per LYG to be unacceptably high and uncertain. The PBAC did not consider the analysis of the post-capecitabine subgroup to be informative, given that the appropriate place of eribulin could be before or after capecitabine and that eribulin could be used in combination with trastuzumab. The PBAC considered that the submission had not adequately addressed the comparative disutility associated with eribulin treatment.
The PBAC also considered that the submission’s estimates of usage and financial implications were uncertain.
The PBAC noted the consumer comments received in relation to the submission.
The PBAC noted that the submission is eligible for an Independent Review.
Recommendation:
Rejected
13. Context for Decision
The PBAC helps decide whether and, if so, how medicines should be subsidised in Australia. It considers submissions in this context. A PBAC decision not to recommend listing or not to recommend changing a listing does not represent a final PBAC view about the merits of the medicine. A company can resubmit to the PBAC or seek independent review of the PBAC decision.
14. Sponsor’s Comment
Whilst Eisai is disappointed with the decision of the PBAC Eisai is committed to working with the PBAC to address the issues and questions that have been raised, to ensure Halaven is listed on the PBS for eligible patients.




