TRASTUZUMAB EMTANSINE, injections, 100 mg vial and 160 mg vial, Kadcyla®, Roche Products Pty Ltd
PDF printable version of this page
1 Purpose of Application
1.1 The major resubmission sought a Section 100 (Efficient Funding of Chemotherapy Drugs) Authority required listing for the treatment of HER2-positive metastatic breast cancer (stage IV) in patients who have received prior therapy with trastuzumab and a taxane, and who meet certain criteria.
2 Requested listing
2.1 The resubmission sought the following listing:
|
Name, Restriction, Manner of administration and form |
Max. Amt |
No. of Rpts |
Proprietary Name |
Manufacturer |
||
|---|---|---|---|---|---|---|
| trastuzumab emtansine Injection |
450 mg |
0
|
Kadcyla
|
RO | ||
Available brands:
Kadcyla
(Trastuzumab emtansine 100 mg injection, 1 x 100 mg vial)
(Trastuzumab emtansine 160 mg injection, 1 x 160 mg vial)
|
Section 100 Efficient Funding of Chemotherapy (Public/Private Hospital)
Authority Required Initial treatment of patients whose disease has progressed despite treatment with trastuzumab for metastatic disease Initial treatment of patients with HER2-positive metastatic breast cancer (stage IV) with a performance status of 0-1, who have received prior therapy with trastuzumab and a taxane and whose disease has progressed despite treatment with trastuzumab for metastatic disease.
Authority applications for initial treatment of trastuzumab emtansine must be made in writing and must include: a) a completed authority prescription form b) a pathology report demonstrating HER2 positivity by in situ hybridization (ISH) c) date of last treatment with a taxane and total number of cycles d) a signed patient acknowledgment e) dates of treatment with trastuzumab for metastatic disease f) date of demonstration of progression whilst on treatment with trastuzumab for metastatic disease.
Authority Required Initial treatment of patients whose disease has progressed during or within 6 months of completing adjuvant trastuzumab therapy Initial treatment of patients with HER2-positive metastatic breast cancer (stage IV) with a performance status of 0-1, who have received prior therapy with trastuzumab and a taxane and whose disease has progressed during or within 6 months of completing adjuvant therapy.
Authority applications for initial treatment of trastuzumab emtansine must be made in writing and must include: a) a completed authority prescription form b) a pathology report demonstrating HER2 positivity by in situ hybridization (ISH) c) date of last treatment with a taxane and total number of cycles d) a signed patient acknowledgment e) dates of adjuvant trastuzumab treatment f) date of demonstration of progression.
Continuing treatment Continuing treatment of patients with HER2-positive metastatic breast cancer (stage IV) who have previously received treatment with PBS subsidised trastuzumab emtansine and who do not have progressive disease. Authority applications must be made in writing and must include: (a) a completed authority prescription form (b) a statement from the prescribing doctor that the disease has not progressed. Note Trastuzumab emtansine should not be used in patients with a left ventricular ejection fraction (LVEF) of less than 40% or with symptomatic heart failure. Cardiac function must be tested by a suitable method including, for example, ECHO or MUGA, prior to seeking the initial authority approval and then at 3 monthly intervals during treatment. |
2.2 The requested listing was based on a cost-utility analysis comparing trastuzumab emtansine to lapatinib administered in combination with capecitabine. Utility was measured in terms of quality-adjusted life-years (QALYs) gained.
2.3 The PBAC noted that the requested PBS listing differed from the previous submission in the following ways:
- a grandfather clause was not included;
- an ECOG performance status must be 0-1 was included;
- HER2 status determined by ISH was included; and
- a NOTE stating that the drug should not be used in patients with symptomatic heart failure, and that cardiac testing should be performed, was included.
These changes were consistent with the July 2013 PBAC minutes.
2.4 The requested NOTE also stated that trastuzumab emtansine should not be used in patients with LVEF < 40%. The PBAC noted that this was not consistent with the EMILIA trial, which required that LVEF ≥ 50%, or the July 2013 PBAC minutes, which recommended that trastuzumab should not be used in patients with LVEF < 45%. However, it was consistent with the TGA product information.
2.5 The requested PBS listing also no longer included the treatment of HER2-positive unresectable locally advanced breast cancer. The PBAC noted that this was consistent with the TGA indication and the financial estimates, but not consistent with the trial population in EMILIA.
2.6 The PBAC considered that in practice, there is high potential for patients with HER2-positive metastatic breast cancer to continue with at least one HER2 blocking medicine, including, trastuzumab, lapatinib, pertuzumab and trastuzumab emtansine (TDM1) until death, despite the proposed restrictions disallowing use in progressive disease. This practice is due to the effectiveness and tolerability of the HER2 targeted medicines compared with conventional cytotoxics and also the usage of trastuzumab on the Herceptin Program. Analysis of drug utilisation on the Herceptin program shows that prescribing does not align with either the TGA registered indications or the restrictions of the Herceptin program. Contemporary practice involves multiple lines of HER2 blocking therapy, and increasing use of dual HER2 blockade. These practices have evolved to achieve additional marginal benefits with varying effects on toxicity. Other than patients with heart failure, very few individuals with HER2 positive breast cancer are not offered HER2 blockade. In relation to efficacy, the trial evidence suggests that when given initially, trastuzumab added to chemotherapy increases overall survival by about six months. The availability of new HER2 blocking agents enables treatment with dual or sequential HER2 blockade. The impact on survival of these new agents depends on factors such as patient population, previous exposure to trastuzumab and presence of CNS disease. In trials, the comparative benefit over the control arm was as follows; pertuzumab (CLEOPATRA trial) showed a 6.1 months delay in progression free survival, lapatinib (EGFR104900) showed a 4.5 month overall survival gain and trastuzumab emtansine (EMILIA) a 5.8 months survival gain. The PBAC understood that since HER2 blockade is maintained from diagnosis to death, increasing treatment exposure will be associated with a diminishing marginal return (measured in QALYs gained) for HER2 treatment compared with chemotherapy/best supportive care.
2.7 To mitigate the financial risk of patients continuing on treatment despite having progressive disease, the PBAC proposed an option whereby the full range of these HER2 blocking medicines (including trastuzumab emtansine) is subsidised through the PBS with an annual cap on their Government expenditure. This was on the provision that drug usage estimates are performed accurately. This estimate would need to consider the established incidence of 30-40 new patients per month and the rise in the current static prevalence of about 1300 patients on trastuzumab. The rise in prevalence could be predicted on the basis of the expected increase in overall survival with the availability of newer HER2 blocking medicines. This approach would also accommodate the advent of subcutaneous trastuzumab in the near future.
2.8 The PBAC acknowledged that the availability of multiple new agents for HER2 positive breast cancer redefined the condition as a chronic disease more analogous to continuous medicinal therapy to manage cardiovascular disease. Given the epidemiological projections above (REDACTED), the current annual expenditure on trastuzumab of about $60 million for the treatment of metastatic disease could realistically be expected to be greatly exceeded.
2.9 The PBAC considered that establishing cost-effectiveness for the suite of HER2 blocking medicines (i.e. pertuzumab, trastuzumab emtansine, trastuzumab, and lapatinib) would allow for implementation of a simplified Authority Required restriction whereby the indication is HER2 positive metastatic breast cancer. The only clinical, treatment or population criterion would be the need to demonstrate HER2 positivity.
2.10 The PBAC noted that clinical guidelines from the European Society for Medical Oncology, American Society of Clinical Oncology, and National Comprehensive Cancer Network now recommend that clinicians re-evaluate hormone receptor and HER2 status at least once in a metastatic lesion. The PBAC therefore considered that a pathology report demonstrating HER2 positivity specific to metastatic disease be included in the initial restrictions before agents for first line HER2 blockade are used in the metastatic setting. This means HER2 positivity in an early stage cancer would not be acceptable evidence of HER2 positivity of metastatic disease. The PBAC noted that this may require a re-examination the role and cost of re-biopsy when repeating HER2 status. The PBAC also agreed that any restriction requiring HER2 testing for future patients with metastatic breast cancer specify the HER2 test be done in a metastatic lesion by in-situ hybridization (ISH) as opposed to immunohistochemistry methods. The role and cost of re-biopsy to repeat HER2 status should be considered further.
3 Background
3.1 Trastuzumab emtansine was TGA registered on 3 September 2013 for the treatment of patients with HER2-positive metastatic (stage IV) breast cancer who previously received trastuzumab and a taxane, separately or in combination. Patients should have either received prior therapy for metastatic disease or, developed disease recurrence during or within six months of completing adjuvant therapy.
3.2 The PBAC had previously considered trastuzumab emtansine once.
3.3 In July 2013, the PBAC considered a submission seeking a Section 100 listing for trastuzumab emtansine for the treatment of a patient with HER2-positive unresectable locally advanced or metastatic breast cancer who has received prior therapy with trastuzumab and a taxane and whose disease has progressed despite treatment with trastuzumab for metastatic disease, or within 6 months of completing adjuvant therapy. The PBAC rejected the submission on the basis of an incorrect comparator. The PBAC considered that the correct comparator was lapatinib plus capecitabine, and noted that the ICER against this regimen was unacceptably high.
4 Clinical place for the proposed therapy
4.1 In Australia, trastuzumab has become the standard treatment for women with HER2-positive breast cancer in the metastatic and early breast cancer setting. HER2 positivity has been associated with poor prognosis and reduced overall survival. While a proportion of patients diagnosed with HER2-positive breast cancer who receive trastuzumab will no longer go on to develop more advanced forms of the disease, there continues to be a clinical need for additional treatment options for those patients whose disease progresses to metastatic breast cancer. Trastuzumab emtansine is a HER2-targeted antibody drug conjugate that contains the humanised anti-HER2 IgG1, trastuzumab, covalently linked to the microtubule inhibitory drug, emtansine.
4.2 Trastuzumab emtansine would provide another treatment option for patients who need an additional treatment following trastuzumab. There are no differences in the clinical place for the proposed therapy compared to the previous submission.
4.3 The PBAC agreed with the ESC that the treatment algorithm for metastatic breast cancer and the cost effectiveness of subsequent therapies would be impacted by both the submission for first-line pertuzumab + trastuzumab combination treatment (PBAC agenda item 5.12 of this meeting) and this resubmission for second-line trastuzumab emtansine. The proposed cost-effectiveness of new treatments should be considered in the context of the entire treatment algorithm.
4.4 The table below provides examples of the different lines of therapies involving HER2-targeted therapies.
|
|
Trastuzumab naïve or sensitive i.e. relapse from adjuvant treatment >1 year |
Trastuzumab pre-treated and early relapse <1 year (or maybe 6 months) |
|---|---|---|
|
1st line |
Docetaxel + trastuzumab + pertuzumab |
Trastuzumab emtansine |
|
2nd line |
Trastuzumab emtansine |
Lapatinib + capecitabinea |
|
3rd line |
Lapatinib + capecitabine |
Lapatinib + trastuzumab |
|
4th line |
Lapatinib + trastuzumaba |
Trastuzumab + chemotherapyb |
|
5th line |
Trastuzumab + chemotherapyb |
|
a Lapatinib+trastuzumab is not yet an approved use, but clinical trials (such as EGF 104900) suggest that the combination is effective and therefore would be likely to be used in future practice.
b Chemotherapy = vinorelbine, capecitabine, gemcitabine, carboplatin, any taxane
5 Comparator
5.1 The resubmission nominated lapatinib plus capecitabine as the comparator. The PBAC considered this to be the appropriate comparator, consistent with its previous advice. The PBAC noted that this was different from the previous submission, which used a mixed comparator of lapatinib plus capecitabine, trastuzumab plus chemotherapy, and, trastuzumab monotherapy.
6 PBAC consideration of the evidence
Consumer comments and sponsor hearing
6.1 The sponsor requested a hearing for this item. The clinician presenting at the hearing spoke in relation to both the pertuzumab and trastuzumab emtansine submissions and noted the enthusiasm amongst clinicians for the listing of these new oncology medicines and discussed the benefits of the new treatments as:
- causing less toxicity (diarrhoea);
- for the few patients who may experience thrombocytopenia side-effects, the clinician considered that these are relatively easy to manage in practice.
6.2 Clarification was sought from the clinician on whether HER2 re-testing is performed in practice. The clinician confirmed that re-testing is performed to ensure that the same cancer (or at least a HER2-positive cancer) is being treated to avoid prescribing the medicine incorrectly.
6.3 The sponsor also confirmed that a sub-cutaneously administered trastuzumab was under development and stated that this was not related to evergreening the patent on trastuzumab.
6.4 The PBAC noted and welcomed the input from individuals (5), health care professionals (7) and organisations (2) via the Consumer Comments facility on the PBS website. The comments described a range of benefits of second-line treatment with trastuzumab emtansine including improved survival, better quality of life, acceptable tolerability with respect to side effects, and, increased equity with subsidised access. The PBAC noted the support for listing and comments on comparative effectiveness/safety and clinical need received from the Breast Cancer Network Australia and the Medical Oncology Group of Australia (MOGA).
Clinical trials
6.5 The resubmission was based on one direct (head-to-head) randomised trial comparing trastuzumab emtansine to lapatinib plus capecitabine in 991 patients with locally advanced breast cancer/metastatic breast cancer or progressed within six months after completing adjuvant therapy: EMILIA. This was a sub-set of the trials presented in the previous submission. The EMILIA trial included patients with locally advanced breast cancer and fast-relapsing patients (proportion unknown). Locally advanced breast cancer patients, who were not also fast-relapsing patients, were excluded from the requested PBS listing.
6.6 Publication details of the EMILIA trial are shown in the table below.
|
Trial ID/ first author |
Protocol title/ Publication title |
Publication citation |
|---|---|---|
|
EMILIA |
Clinical Study Report - TDM4370g (BO21977) - EMILIA. A randomized, multicenter, phase III open-label study of the efficacy and safety of trastuzumab emtansine vs. capecitabine + lapatinib in patients with HER2-positive locally advanced or metastatic breast cancer who have received prior trastuzumab-based therapy. Report No. 1044311.
|
August 2012 |
|
Blackwell |
Primary results from EMILIA, a phase III study of trastuzumab emtansine (T DM1) versus capecitabine (X) and lapatinib (L) in HER2-positive locally advanced or metastatic breast cancer (MBC) previously treated with trastuzumab (T) and a taxane.
|
J Clin Oncol 2012 30: (Suppl; abstr LBA1). ASCO Annual Meeting, 2012. |
|
Pegram |
Primary results from EMILIA, a phase III study of trastuzumab emtansine (T-DM1) versus capecitabine (X) and lapatinib (L) in HER2-positive locally advanced or metastatic breast cancer (MBC) previously treated with trastuzumab (T) and a taxane.
|
J Clin Oncol 2012; 30:27 SUPPL. |
|
Verma |
EMILIA: A phase III, randomized, multicenter study of trastuzumab-DM1 (T-DM1) compared with lapatinib (L) plus capecitabine (X) in patients with HER2-positive locally advanced or metastatic breast cancer (MBC) and previously treated with a trastuzumab-based regimen.
|
J Clin Oncol 2011; 29 (suppl; abstr TPS116). ASCO Annual Meeting, 2011. |
|
Verma |
Updated overall survival results from EMILIA, a phase 3 study of trastuzumab emtansine (T-DM1) vs. capecitabine and lapatinib in HER2-positive locally advanced or metastatic breast cancer.
|
Presentation at ESMO 2012 Congress; Vienna, Austria. |
|
Verma |
Trastuzumab emtansine for HER2-positive advanced breast cancer. |
N Engl J Med 2012 Nov 8 (Epub 2012 Oct 1); 367(19):1783-91
|
|
Wang |
Exposure-efficacy relationship of trastuzumab emtansine (T-DM1) in EMILIA, a phase III study of T-DM1 versus capecitabine (X) and lapatinib (L) in HER2-positive locally advanced or metastatic breast cancer (MBC).
|
J Clin Oncol 2013; 31:15 SUPPL. 1. |
|
Wang |
Pharmacokinetics and exposure-efficacy relationship of trastuzumab emtansine in EMILIA, a phase 3 study of trastuzumab emtansine vs. capecitabine and lapatinib in HER2-positive locally advanced or metastatic breast cancer.
|
Cancer Research 2012; 72:24 SUPPL. 3. |
|
Welslau |
Patient-reported outcomes from EMILIA, a phase 3 study of trastuzumab emtansine (T-DM1) vs. capecitabine and lapatinib (XL) in HER2-positive locally advanced or MBC. |
Poster presentation 329P. ESMO 2012 Congress; Vienna, Austria.
|
|
Weslau |
Patient-reported outcomes from EMILIA, a randomized phase 3 study of trastuzumab emtansine (T-DM1) versus capecitabine and lapatinib in human epidermal growth factor receptor 2-positive locally advanced or metastatic breast cancer. |
Cancer. November 2013. Epub ahead of print. |
Source: B.2.4, p10, Section B of the resubmission and compiled during the evaluation.
Comparative effectiveness
6.7 The comparative effectiveness was unchanged from the previous submission. Based on EMILIA, trastuzumab emtansine is associated with a statistically significant longer progression-free survival (PFS) and overall survival (OS) compared to lapatinib plus capecitabine.
6.8 The main PFS and OS results are summarised in the table below and the following two figures:
|
Trial ID |
Trastuzumab emtansine Median months (95% CI) |
Lapatinib + capecitabine Median months (95% CI) |
Absolute Difference |
HR (95% CI)# |
|---|---|---|---|---|
|
PFS (IRC assessed) as at 14 January 2012 |
9.6 (REDACTED) (N=495) |
6.4 (REDACTED) (N=496) |
3.2 |
0.65 (0.59, 0.77) P<0.0001 |
|
OS as at 14 January 2012 |
NE (REDACTED) (N=495) |
23.3 (REDACTED) (N=496) |
NE |
0.62 (0.48, 0.81) P=0.0005 |
|
OS as at 31 July 2012 |
30.9 (REDACTED) (N=495) |
25.1 (REDACTED) (N=496) |
5.8 |
0.68 (0.55, 0.85) P=0.0006 |
#stratified analysis. CI: confidence interval; IRC: independent review committee; HR: hazard rate ratio; N: number of patients; NE: not evaluable; OS: overall survival; PFS: progression-free survival. Source: Table B.6.4-5 p49-50, Section B of the resubmission and calculated during the evaluation (in italics).
Kaplan-Meier plot of IRC-assessed PFS in the EMILIA trial (14 January 2012)
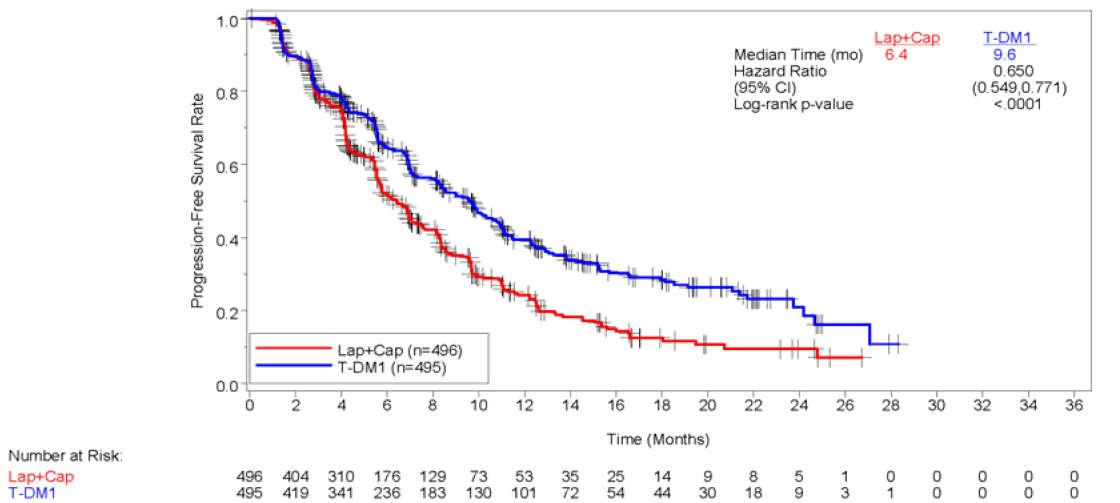
Source: Figure B.6.1 p49 or Section B of the resubmission.
Kaplan-Meier plot of OS in the EMILIA trial (31 July 2012)
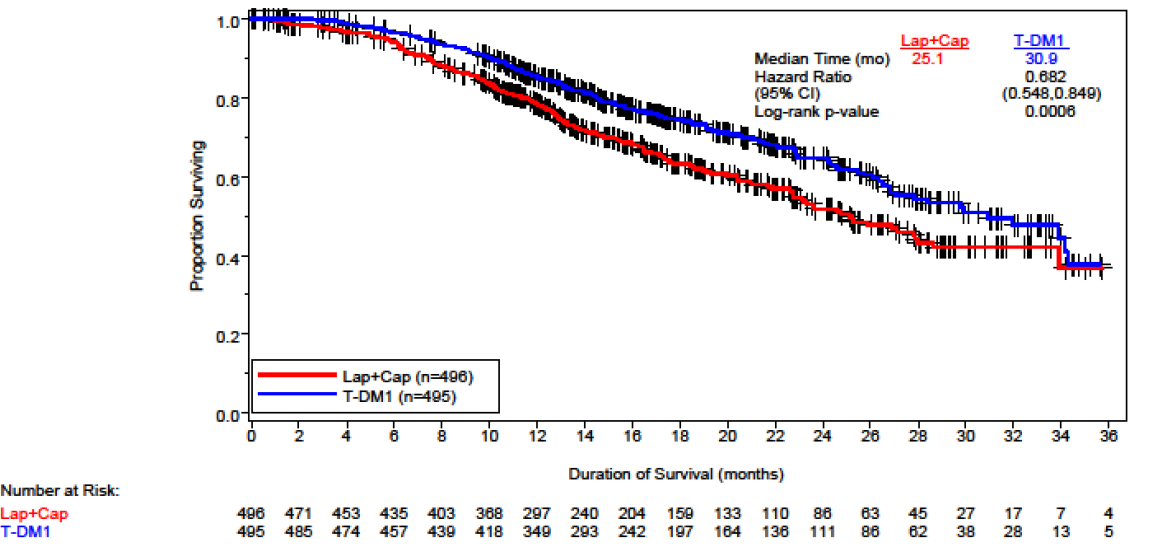
Source: Figure B.6.3, p53, Section B of the resubmission.
6.9 The PBAC noted that, with a median PFS improvement of 3.2 months and a median OS improvement of 5.8 months, there is evidence of benefit in the post-progression period after trastuzumab emtansine is ceased. The model extrapolates this to (REDACTED) life-years gained ((REDACTED) months), of which (REDACTED) years ((REDACTED) months) is progression-free and (REDACTED) years ((REDACTED) months) is in progression.
6.10 Further sub-group analyses of IRC-assessed PFS and OS gains found that patients who had not received prior systemic therapy for metastatic breast cancer (i.e. fast-relapsing patients) benefited slightly more than those who had received prior systemic therapy for metastatic breast cancer although the difference is unlikely to be statistically significant:
- PFS: Fast-relapsing patients HR=0.51 (95%CI: 0.30, 0.85) vs. other patients HR=0.69 (95%CI: 0.58, 0.82
- OS: Fast-relapsing patients HR=(REDACTED) (95%CI: (REDACTED)) vs. other patients HR=(REDACTED) (95%CI: (REDACTED)).
6.11 Sub-group analyses of health outcomes by locally advanced breast cancer versus metastatic breast cancer were not reported in the clinical study report.
6.12 The resubmission also presented estimates of overall response rate (ORR), clinical benefit rate (CBR), investigator-assessed PFS, duration of response, time to treatment failure (TTF), and time to symptom progression:
- IRC- and investigator-assessed ORR was significantly larger with trastuzumab emtansine. Most patients treated with trastuzumab emtansine experienced a partial response (97.7% of responders) rather than a complete response.
- Investigator-assessed PFS and time to TTF were significantly longer with trastuzumab emtansine.
- Duration of response was longer (statistical significance not tested) in EMILIA.
- Time to symptom progression was significantly longer with trastuzumab emtansine (measured using the FACT-B TOI-PFB[1]).
6.13 The PBAC also noted there was 10% greater use of HER2 therapy (52% versus 61%) following progression with trastuzumab emtansine compared with lapatinib and capecitabine, with 48% of patients receiving lapatinib after trastuzumab emtansine. The total duration or number of cycles of post-progression treatment until death was not reported for each of the trial arms. The trial data indicates that HER2 blockade continues beyond progression, with a greater opportunity for post-progression costs following trastuzumab emtansine.
Comparative harms
6.14 The comparative harms are unchanged from the previous submission (see summary table below. Based on EMILIA, trastuzumab emtansine was associated with more cases of hepatotoxicity, thrombocytopenia/bleeding, infusion reactions, fatigue, constipation, arthralgia; but fewer cases of gastrointestinal toxicity, mucosal inflammation, hand foot syndrome and rash.
6.15 In terms of treatment related grade ≥ 3 AEs, trastuzumab emtansine was associated with statistically significantly more cases of hepatotoxicity and thrombocytopenia, and statistically significantly fewer cases of gastrointestinal toxicity, mucosal inflammation, and hand foot syndrome. The overall incidence of cardiac dysfunction and decreases in LVEF was also low. Trastuzumab emtansine was associated with more frequent decreases in LVEF but fewer cases of cardiac dysfunction (all cases were asymptomatic (grade 1 or 2) declines in LVEF).
6.16 The resubmission also reported additional information on the Diarrhoea Assessment Scale (DAS). The proportion of patients with diarrhoea symptoms was consistently greater with lapatinib plus capecitabine versus trastuzumab emtansine, which remained near baseline levels through to cycle eight. The resubmission claimed that this indicated that the number of patients reporting diarrhoea symptoms remained constant over time while on treatment, supporting the assumption that disutilities continue until progression. The ESC advised that this claim was reasonable with respect to diarrhoea.
6.17 The resubmission also reported additional information on the FACT-B PWB[2] subscale. Analysis of this data found there was a clinically meaningful and statistically significant improvement in the item ‘Bothered by side effects’ for patients in the trastuzumab emtansine arm compared with patients in the lapatinib plus capecitabine arm through week 24.
6.18 A summary of the key adverse events in the EMILIA trial is shown in the table below.
|
|
T-DM1 |
Lapatinib + capecitabine |
RR (95%CI) |
(REDACTED) |
(REDACTED) |
|---|---|---|---|---|---|
|
N |
490 |
488 |
|
(REDACTED) |
(REDACTED) |
|
Any AE |
470 (95.9%) |
477 (97.7%) |
0.981 (0.959, 1.004) |
(REDACTED) |
(REDACTED) |
|
Treatment related AE grade ³3 |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
|
AE grade ³3 |
200 (40.8%) |
278 (57.0%) |
0.716 (0.628, 0.817) |
(REDACTED) |
(REDACTED) |
|
SAE |
76 (15.5%) |
88 (18.0%) |
0.860 (0.650, 1.138) |
(REDACTED) |
(REDACTED) |
|
Death |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
|
Death due to PD |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
|
Death due to causes other than PD |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
|
AE leading to discontinuation |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
|
(REDACTED) |
(REDACTED) |
||||
|
AE leading to dose reduction |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
|
(REDACTED) |
(REDACTED) |
||||
|
AE leading to dose delay of T-DM1 |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
|
Diarrhoea SAEs |
(REDACTED) |
(REDACTED) |
|
(REDACTED) |
(REDACTED) |
|
Thrombocyto-penia SAEs |
(REDACTED) |
(REDACTED) |
|
(REDACTED) |
(REDACTED) |
AE: adverse event; C: capecitabine; L: lapatinib; NA: not applicable; NR: not reported; SAE: serious adverse event; PD: Progressive disease; RR: relative risk; T-DM1: trastuzumab emtansine. Source: Table B.6.14 p65, Tables B.6.28-29 p84-85, of Section B of the resubmission, and Table B.6.34-6, p119-25, of Section B of the previous submission and calculated during the evaluation. The relative risks have been verified as correct.
6.19 The resubmission provided additional data on potential safety concerns beyond those identified in the clinical trials. No new AEs were identified and the incidence of AEs was generally similar in the pooled group of single arm studies compared to EMILIA.
6.20 A summary of the comparative benefits and harms for trastuzumab emtansine versus lapatinib plus capecitabine is presented in the table below.
|
Outcome |
N |
HR or RR (95% CI) |
Median months (95% CI)a |
Increment |
|
|---|---|---|---|---|---|
|
Trastuzumab emtansine |
Lapatinib + capecitabine |
||||
|
Benefits |
|||||
|
PFS (IRC assessed) as at 14 January 2012 |
991 |
0.65 (0.59, 0.77)# |
9.6 (REDACTED) |
6.4 (REDACTED) |
3.2 |
|
OS as at 31 July 2012 |
991 |
0.68 (0.55, 0.85)# |
30.9 (REDACTED) |
25.1 (REDACTED) |
5.8 |
|
|
Proportions |
|
|||
|
Alive as at 31 July 2012 |
|
|
(REDACTED) |
(REDACTED) |
(REDACTED) |
|
Harms |
|
|
|||
|
All grade³3 AEs |
978 |
0.716 (0.628, 0.817) |
40.8% |
57.0% |
-16.2% |
|
Grade ³3 thrombocytopenia |
978 |
62.743 (8.737, 450.590) |
12.9% |
0.2% |
12.7% |
|
Grade ³3 diarrhoea |
978 |
0.079 (0.039, 0.160) |
1.6% |
20.7% |
-19.1% |
|
Grade ³3 vomiting |
978 |
0.181 (0.063, 0.522) |
0.8% |
4.5% |
-3.7% |
#stratified analysis. a For duration of EMILIA (median follow-up 12.65 months for PFS and 18.85 months for OS). CI: confidence interval; IRC: independent review committee; HR: hazard rate ratio; N: number of patients; OS: overall survival; PFS: progression-free survival; Source: Table B.6.4-5 p49-50 and B.6.29 p85, Section B of the resubmission and calculated during the evaluation.
The PBAC also noted that, based on the results from the table above, for every 100 patients treated with trastuzumab emtansine compared to lapatinib plus capecitabine:
- approximately 10 more patients would be alive 36 months (3 years) after the start of treatment
- approximately 16 less patients would experience any grade 3 or higher adverse event
- approximately 13 more patients would experience the adverse event of thrombocytopenia
- approximately 19 less patients would experience the adverse event of diarrhoea
- approximately 4 less patients would experience the adverse event of vomiting.
Clinical claim
6.21 The resubmission described trastuzumab emtansine as superior in terms of comparative effectiveness and superior in terms of comparative safety over lapatinib plus capecitabine.
6.22 The PBAC accepted this clinical claim, although noted that some of the toxicity profile of trastuzumab emtansine was less favourable than that of its comparator
Economic analysis
6.23 The economic evaluation was largely the same as in the previous submission. The key changes were: lapatinib plus capecitabine was the only comparator (and thus the indirect comparison in the previous submission was no longer relevant); OS was based on the second data cut-off in EMILIA (18.85 months); a new risk sharing arrangement (REDACTED)) was included; a 10% price reduction for lapatinib was no longer assumed; and post-progression treatment costs and a shorter time horizon were considered in the sensitivity analysis.
6.24 (REDACTED)' the Kaplan-Meier estimates of Time to Off-Treatment (TTOT) from the first-interim analysis (14 January 2012) were used up until the pooled median follow-up times from EMILIA (12.65 months or 54.8 weeks), after which TTOT was extrapolated using the fitted model for PFS.
6.25 (REDACTED)
6.26 (REDACTED)
6.27 The resubmission calculated an incremental cost/extra QALY gained in the range of $45,000 - $75,000 (vs. lapatinib plus capecitabine).
6.28 The PBAC noted that the incremental cost-effectiveness ratio (ICER) (vs. lapatinib + capecitabine) was lower than the previous submission ($45,000 - $75,000– this resubmission vs. $75,000 - $105,000– July 2013 submission). This was mainly because the new proposed risk sharing arrangement (REDACTED) was included, and a 10% price reduction for lapatinib was no longer assumed.
6.29 The PBAC noted that the steps in the economic model that had large effects on the ICER were: Step 2, where PFS, OS and TTOT were extrapolated using a gamma function; and Step 6 where a risk-sharing arrangement (REDACTED) was applied. The PBAC did not consider that it would be feasible to implement such a risk-sharing arrangement and noted that this arrangement would not achieve its intended risk-sharing effect unless it applied to use of the medicine for the remainder of each patient’s life. Without this arrangement, the ICER increases to $105,000 to $200,000/QALY.
6.30 Univariate sensitivity analyses were conducted. The PBAC noted that the ICER was most sensitive to a shorter time horizon (the ICER increased to between $45,000 to $75,000/QALY), parametric extrapolation of PFS using a Weibull function (the ICER increased to between $75,000 to $105,000), and the mean dose received or patient’s weight (the ICER increased into a range of $75,000 to $105,000 if 3 x 100 mg vials of trastuzumab emtansine were required).
6.31 The PBAC noted that the sensitivity analysis regarding the inclusion of post-progression costs reported by the resubmission (decreased to a range of $15,000 to $45,000) did not include ongoing costs after third-line therapy (additional lines of therapy, specialist visits, etc). The post-progression treatment costs were estimated as a one-off cost at progression for this sensitivity analysis:
- The average cost of post-progression therapy following trastuzumab emtansine was assumed to be third-line lapatinib plus capecitabine having the same cost/patient as estimated in second-line (i.e. $50,000), including drug, administration, medical resource use and adverse event costs – the proportion of patients continuing on to fourth-line trastuzumab plus chemotherapy would need to be estimated.
- The average cost of post-progression therapy following lapatinib plus capecitabine was assumed to be third-line trastuzumab plus chemotherapy based on the costs estimated in the model submitted in the previous submission (i.e. $69,000) – if the (REDACTED) trastuzumab price reduction is included, these costs reduced to (REDACTED) and the ICER increased from between $15,000/QALY and $45,000/QALY to between $15,000/QALY and $45,000/QALY, which was still less than the base case.
- Post-progression costs were therefore implausibly estimated to be $19,000 less following trastuzumab emtansine for this sensitivity analysis.
6.32 The PBAC agreed with the ESC that the problem with the estimate of post-progression costs was a particular example of the importance of considering the entire treatment algorithm. Despite the model estimating that the post progression state (REDACTED) accounted for approximately 35%-40% of the incremental survival gain with trastuzumab emtansine (REDACTED), the model assumed that this health state either contributed no extra costs (base case) or cost off-sets (sensitivity analysis). The PBAC noted the following particular concerns with this aspect of the model:
- when included, post-progression costs were assumed to occur only at the time of progression, which did not account for the estimated difference in time in this state ((REDACTED) years with trastuzumab emtansine and (REDACTED) years with the comparator)
- when included, post-progression costs were for only one extra line of therapy, which is not consistent with Australian practice to move on to later lines of therapy (for example the recent change in the PBS restriction for lapatinib to allow patients to return to trastuzumab-based therapy)
- (REDACTED)
- a greater proportion of patients were likely to receive post-progression therapy after trastuzumab emtansine than its comparator - results from the EMILIA trial indicate that, after discontinuation of the study treatment, 61% of the trastuzumab emtansine arm of the EMILIA trial received a HER2 targeted therapy compared to 52% of the lapatinib and capecitabine arm, with 18% of the trastuzumab emtansine arm subsequently receiving both trastuzumab and lapatinib compared with 8% of the lapatinib and capecitabine arm.
6.33 The PBAC considered that failing to account adequately for extra post-progression costs with trastuzumab emtansine compared with its comparator inappropriately reduced the estimated ICER in favour of trastuzumab emtansine. The PBAC considered that an estimate of costs proportional to the time in the progressive disease health state until death would be more realistic, noting the evidence of use of ongoing HER2 blockade until death. Modelling based on data on post-progression treatment sequence and duration would form a stronger basis for costing the progressive disease health state.
6.34 The long-term cost-effectiveness of trastuzumab emtansine is therefore affected by the potential availability of other HER2 agents on the PBS. Therefore as noted above under ‘Clinical place for the proposed therapy’, the PBAC considered that the proposed cost-effectiveness of new treatments in HER2-positive metastatic breast cancer should be considered in the context of the entire treatment algorithm, including dual and sequential HER2 blockade.
6.35 Overall, the PBAC considered the trastuzumab emtansine economic model as presented in the submission was reasonably reliable despite the issues outlined above and the potential for the ICER to be underestimated. However, PBAC again noted their preference for assessing the cost-effectiveness of the suite of anti-HER2 agents as they would be used in contemporary clinical practice. For this reason, PBAC considered it difficult to interpret the trastuzumab emtansine model in isolation from the models for other anti-HER2 drugs.
6.36 In view of the improved survival benefit of trastuzumab emtansine the PBAC recommended the Department engage with the sponsors of all anti-HER2 agents, professional oncology groups and consumers to progress the potential PBS listings of all medicines to support contemporary, evidence based clinical practice. This engagement should be independent of the type of HER2 blockade; account for the diminishing marginal effect of HER2 blockade with longer duration of exposure; and acknowledge that switching to a new HER2 blocking regimen usually signifies failure of the existing regimen.
6.37 The PBAC proposed that the basis for this engagement should be to manage HER2-positive metastatic breast cancer over the remainder of the lifetime of an eligible patient, with an expenditure cap across this population. This would need to address the use of trastuzumab funded via the Herceptin Program, the use of trastuzumab as monotherapy and its use in combination with other cytotoxics. (REDACTED). Subcutaneous trastuzumab would need to match the price of intravenous trastuzumab at all times.
6.38 The PBAC advised that this engagement should also work towards revised financial estimates across all HER2 blocking medicines in metastatic breast cancer over the next five years. This should be informed by:
- the speed of uptake of the Herceptin Program (about 4 years to reach plateau);
- a 2.3% increase in the annual incidence of metastatic breast cancer;
- realistic estimates of the duration of therapy with each medicine over the average lifetime of a patient, including overlapping periods where they are given in combination;
- and a more realistic assumption of continuous HER2 blockade for the remainder of the average life expectancy.
The revised calculations should estimate:
- the number of patients at steady state;
- when steady state will be reached;
- and the duration of exposure to each HER2 agent over the life time of a patient (on average with 95% CI).
This would form the basis of a cap on annual expenditure for each medicine, to be imposed with a 100% rebate for expenditure beyond its cap.
6.39 The PBAC would welcome submissions which reflected these arrangements and provide economic evaluations which informed the cost-effectiveness of the component HER2 blocking medicines within the arrangements. Although all three models (for trastuzumab, pertuzumab and trastuzumab emtansine) need to account more realistically for post-progression use, the PBAC generally accepted the structures of the models.
6.40 The PBAC further advised that any implemented arrangement should be subject to data collection and analysis funded by the sponsors of the medicines. This should examine: the duration of exposure to each HER2 agent; sequences of use; co-administered therapies, re-cycling of medicines, treatment patterns in relation to exposure to adjuvant trastuzumab. The analysis should be conducted and reported on an annual basis.
Estimated PBS usage & financial implications
6.41 This resubmission was not considered by DUSC.
6.42 The net cost to PBS was estimated to be between $100 million and $150 million over 5 years with savings to the Herceptin Program between $50 million and $100 million over 5 years
|
|
Year 1 |
Year 2 |
Year 3 |
Year 4 |
Year 5 |
|---|---|---|---|---|---|
|
Estimated extent of use |
|||||
|
Number treated |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
|
Number treated, previous submission |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
|
Prescriptionsa |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
|
Prescriptionsa, previous submission |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
|
Estimated net cost to PBS/RPBS/MBS ($) |
|||||
|
Net cost to PBSb |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
|
Net cost to MBS |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
|
Estimated total net cost |
|||||
|
Totalb |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
|
Estimated net cost to PBS/RPBS/MBS, previous submission ($) |
|||||
|
Net cost to PBS |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
|
Net cost to MBS |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
|
Estimated total net cost - July 2013 ($) |
|||||
|
Totalc |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
a (REDACTED). Source: Table E.2.3 p18, Table E.2.7 p20, Table E.4.1 p38, Table E.5.15 p51, Table E.5.18 p54, Section E of the resubmission and Table E.2.3 p15, Table E.2.7 p18, Table E.4.1 p38, Table E.5.14 p51, Table E.5.17-8 p55, Section E of the previous submission.
6.43 The ESC advised that there was potential for the net cost/year to the PBS to be more than the estimate in the resubmission if lapatinib plus capecitabine is used in third-line following progression on trastuzumab emtansine. On the other hand, the ESC advised that there was potential for the net cost/year for the PBS to be less than the estimate in the resubmission as it did not consider the impact of patients developing poor LVEF or symptomatic heart failure with trastuzumab or not electing to receive further chemotherapy in the estimates of the number of patients, and the maximum of six cycles of cytotoxics in combination with trastuzumab (especially capecitabine). Moreover, due to variation around the mean dose received and patients’ weight, some patients would require 3x100 mg vials rather than 1x100 mg and 1x160 mg vials.
6.44 The PBAC noted that a new risk-sharing arrangement was proposed (REDACTED). The ESC advised that care would need to be taken in finalising any RSA, both in terms of maintaining the intended effect on the ICER and of minimising unnecessary administrative burden.
6.45 (REDACTED)
7 PBAC Outcome
7.1 The PBAC deferred making a recommendation on listing trastuzumab emtansine to enable it to first consider, establish and accept the cost-effectiveness of trastuzumab for the treatment of metastatic breast cancer, before making a judgement on the cost-effectiveness of all HER2 blocking medicines in metastatic breast cancer, including trastuzumab emtansine.
Outcome:
Deferred
Context for Decision
The PBAC helps decide whether and, if so, how medicines should be subsidised in Australia. It considers submissions in this context. A PBAC decision not to recommend listing or not to recommend changing a listing does not represent a final PBAC view about the merits of the medicine. A company can resubmit to the PBAC or seek independent review of the PBAC decision.
Sponsor’s Comment
Roche is disappointed by the decision of the PBAC not to recommend KADCYLA for inclusion on the PBS based on this resubmission. Roche has carefully considered the PBAC’s commentary and is working with the Committee and stakeholders to address outstanding issues of concern such that this much needed medicine can be PBS listed at the earliest possible opportunity.
[1] Functional Assessment of Cancer Therapy – Breast cancer, Trial Outcomes Index-Physical/Functional/Breast
[2] Functional Assessment of Cancer Therapy – Breast cancer, Physical Well Being




