PERTUZUMAB, 420 mg/14 mL injection, 1 x 14 mL vial Perjeta®, Roche Products Pty Ltd
PDF printable version of this page
1 Purpose of Application
1.1 The major submission sought a Section 100 (Efficient Funding of Chemotherapy Drugs), Authority required listing for pertuzumab, given in combination with trastuzumab and docetaxel, for the treatment of a patient with HER2-positive metastatic breast cancer who has not received prior anti-HER2 therapy or chemotherapy for their metastatic disease.
1.2 The PBAC noted that the request therefore also indirectly sought PBS listing of trastuzumab for metastatic breast cancer, an indication for which trastuzumab is currently subsidised by the Commonwealth in a program separate from the PBS (i.e. the ‘Herceptin® Program’).
2 Requested listing
2.1 The submission’s requested listing is shown below.
|
Name, Restriction, Manner of administration and form |
Max. Amt |
No. of Rpts |
Proprietary Name |
Manufacturer |
||
|---|---|---|---|---|---|---|
|
PERTUZUMAB Injection |
840 mg |
0 |
Perjeta |
RO |
||
Available brands:
Perjeta
(Pertuzumab 420 mg injection, 1 x 420 mg vial)
Section 100 Efficient Funding of Chemotherapy (Public/Private Hospital)
Authority Required
Initial treatment
Initial treatment for HER2-positive metastatic breast cancer commencing in combination with trastuzumab and docetaxel for patients who have not received prior anti-HER2 therapy or chemotherapy for metastatic disease.
Authority applications for initial treatment of Perjeta must be made in writing and must include:
(a) a completed authority prescription form
(b) a pathology report demonstrating HER2 positivity by in situ hybridization (ISH)
(c) a signed patient acknowledgment
|
Name, Restriction, Manner of administration and form |
Max. Amt |
No. of Rpts |
Proprietary Name |
Manufacturer |
|
|---|---|---|---|---|---|
|
PERTUZUMAB Injection |
420 mg |
3 |
Perjeta |
RO | |
Available brands:
Perjeta
(Pertuzumab 420 mg injection, 1 x 420 mg vial)
Section 100 Efficient Funding of Chemotherapy (Public/Private Hospital)
Authority Required
Continuing treatment
Continuing treatment, in combination with trastuzumab, for HER2-positive metastatic breast cancer where the patient has previously received treatment with PBS subsidised pertuzumab and does not have progressive disease.
Authority applications must be made in writing and must include:
(a) a completed authority prescription form and
(b) a statement from the prescribing doctor that the disease has not progressed.
2.2 The submission’s proposed flow-on restriction changes to trastuzumab are shown below.
|
Name, Restriction, Manner of administration and form |
Max. Amt |
No. of Rpts |
Proprietary Name |
Manufacturer |
|
|---|---|---|---|---|---|
|
TRASTUZUMAB Injection |
1,000 mg |
0 |
Herceptin |
RO | |
Available brands:
Herceptin
(Trastuzumab 150 mg injection, 1 x 150 mg vial)
(Trastuzumab 60 mg injection, 1 x 60 mg vial)
Add new indications as follows:
Section 100 Efficient Funding of Chemotherapy (Public/Private Hospital)
Authority Required
Initial treatment
Initial treatment for HER2-positive metastatic breast cancer commencing in combination with pertuzumab and docetaxel for patients who have not received prior anti-HER2 therapy or chemotherapy for metastatic disease.
Authority applications for initial treatment of Herceptin must be made in writing and must include:
(a) a completed authority prescription form
(b) a pathology report demonstrating HER2 positivity by in situ hybridization (ISH)
(c) a signed patient acknowledgment
|
Name, Restriction, Manner of administration and form |
Max. Amt |
No. of Rpts |
Proprietary Name |
Manufacturer |
|
|---|---|---|---|---|---|
|
TRASTUZUMAB Injection |
750 mg |
3 |
Herceptin |
RO |
|
Available brands:
Herceptin
(Trastuzumab 150 mg injection, 1 x 150 mg vial)
(Trastuzumab 60 mg injection, 1 x 60 mg vial)
Section 100 Efficient Funding of Chemotherapy (Public/Private Hospital)
Authority Required
Continuing treatment
Continuing treatment, in combination with pertuzumab, for HER2-positive metastatic breast cancer where the patient has previously received treatment with PBS-subsidised pertuzumab plus trastuzumab and does not have progressive disease.
Authority applications must be made in writing and must include:
(a) a completed authority prescription form and
(b) a statement from the prescribing doctor that the disease has not progressed.
2.3 Listing was sought on a cost-utility basis compared with trastuzumab administered in combination with a taxane.
2.4 There was some confusion between the Commentary and the sponsor’s responses about the proposed target population for the restriction. The PBAC considered that the appropriate target population is 2 groups of patients: those with metastatic disease who have not received anti-HER2 treatment and also those patients who have not received chemotherapy for metastatic disease.
2.5 The pre-sub-committee response (p.3) claimed that the Commentary Section A.2 (5.12.COM.15) incorrectly stated that patients who have previously received trastuzumab for Early Breast Cancer are excluded from the restriction. It was noted that the relevant section of the proposed restriction (shown in underline below):
‘Initial treatment for HER2-positive metastatic breast cancer commencing in combination with pertuzumab and docetaxel for patients who have not received prior anti-HER2 therapy or chemotherapy for metastatic disease’.
Was interpreted as:
Patient must not have received prior anti-HER2 therapy; AND
Patient must not have received prior chemotherapy for metastatic disease
The clinical criteria would have been better stated as follows:
Patient must not have received prior anti-HER2 therapy for metastatic disease; AND
Patient must not have received prior chemotherapy for metastatic disease
2.6 The pre-sub-committee response (p.2) stated that ‘the sponsor accepts the proposed additions to the PBS restriction: WHO performance status ≤ 1, left ventricular ejection fraction (LVEF) ≥ 45% and monitoring of cardiac function’.
2.7 The PBAC considered that other taxane partners (paclitaxel and nab-paclitaxel) are clinically equivalent to docetaxel and it is likely that other taxanes (and possibly other cytotoxic partners) would be used instead of docetaxel to some extent despite the TGA registered indication specifically limiting use of pertuzumab in combination with docetaxel (and trastuzumab).
2.8 The PBAC considered that in practice, there is high potential for patients with HER2-positive metastatic breast cancer to continue with at least one HER2 blocking medicine, including, trastuzumab, lapatinib, pertuzumab and trastuzumab emtansine (TDM1) until death, despite the proposed restrictions disallowing use in progressive disease. This practice is due to the effectiveness and tolerability of the HER2 targeted medicines compared with conventional cytotoxics and also the usage of trastuzumab on the Herceptin Program. Analysis of drug utilisation on the Herceptin program shows that prescribing does not align with either the TGA registered indications or the restrictions of the Herceptin program. Contemporary practice involves multiple lines of HER2 blocking therapy, and increasing use of dual HER2 blockade. These practices have evolved to achieve additional marginal benefits with varying effects on toxicity. Other than patients with heart failure, very few individuals with HER2 positive breast cancer are not offered HER2 blockade. In relation to efficacy, the trial evidence suggests that when given initially, trastuzumab added to chemotherapy increases overall survival by about six months. The availability of new HER2 blocking agents enables treatment with dual or sequential HER2 blockade. The impact on survival of these new agents depends on factors such as patient population, previous exposure to trastuzumab and presence of CNS disease. In trials, the comparative benefit over the control arm was as follows; pertuzumab (CLEOPATRA trial) showed 6.1 months delay in progression free survival, lapatinib (EGFR104900) showed 4.5 months overall survival gain and trastuzumab emtansine (EMILIA) 5.8 months survival gain. The PBAC understood that since HER2 blockade is maintained from diagnosis to death, increasing treatment exposure will be associated with a diminishing marginal return (measured in QALYs gained) for HER2 treatment compared with chemotherapy/best supportive care.
2.9 To mitigate the financial risk of patients continuing on treatment despite having progressive disease, the PBAC proposed an option whereby the full range of HER2 blocking medicines (including pertuzumab) is subsidised through the PBS with an annual cap on their Government expenditure. This was on the provision that drug usage estimates are performed accurately. This estimate would need to consider the established incidence of 30-40 new patients per month and the rise in the current static prevalence of about 1300 patients on trastuzumab. The rise in prevalence could be predicted on the basis of the expected increase in overall survival with the availability of newer HER2 blocking medicines. This approach would also accommodate the advent of subcutaneous trastuzumab in the near future.
2.10 The PBAC acknowledged that the availability of multiple new agents for HER2 positive breast cancer redefined the condition as a chronic disease more analogous to continuous medicinal therapy to manage cardiovascular disease. Given the epidemiological projections above (REDACTED)', the current annual expenditure on trastuzumab of about $60 million for the treatment of metastatic disease could realistically be expected to be greatly exceeded.
2.11 The PBAC did not support the approach proposed in the submission to leave some trastuzumab use in the Herceptin Program, and to move some use of trastuzumab for metastatic breast cancer into the PBS. This would be inequitable for patients (eg patients currently receiving trastuzumab on the Herceptin Program will be unable to access pertuzumab). It would promote poor prescribing regarding decisions about partner cytotoxics. In this regard, it was noted that partner chemotherapies such as vinorelbine and also aromatase inhibitors are frequently used with Trastuzumab. These prescribing practices occurred despite the fact that they were not permitted under the Herceptin Program. Over many years, these issues have been raised by breast cancer clinicians through MOGA. The need to access trastuzumab via two funding schemes would create administrative complexities for clinicians and government alike.
2.12 In the event of a transfer of trastuzumab for metastatic breast cancer to the PBS, the PBAC foreshadowed that the PBS restriction would need to be re-written to reflect current evidence and allow for multiple options of partner chemotherapy and use beyond disease progression.
2.13 The PBAC considered that establishing cost-effectiveness for the suite of HER2 blocking medicines (i.e. pertuzumab, trastuzumab emtansine, trastuzumab, and lapatinib) would allow for implementation of a simplified Authority Required restriction whereby the indication is HER2 positive metastatic breast cancer. The only clinical, treatment or population criterion would be the need to demonstrate HER2 positivity.
2.14 The PBAC noted that clinical guidelines from the European Society for Medical Oncology, American Society of Clinical Oncology, and National Comprehensive Cancer Network now recommend that clinicians re-evaluate hormone receptor and HER2 status at least once in a metastatic lesion. The PBAC therefore considered that a pathology report demonstrating HER2 positivity specific to metastatic disease be included in the initial restrictions for pertuzumab and trastuzumab (i.e. HER2 positivity when the cancer was in an early stage would not be acceptable evidence). The PBAC noted that this may require a re-examination the role and cost of re-biopsy when repeating HER2 status. The PBAC also agreed that any restriction requiring HER2 testing for future patients with metastatic breast cancer specify the HER2 test be done in a metastatic lesion by in-situ hybridization (ISH) as opposed to immunohistochemistry methods. The role and cost of re-biopsy to repeat HER2 status should be considered further.
3 Background
3.1 Pertuzumab was TGA registered on 7 May 2013, to be used in combination with trastuzumab and docetaxel for patients with HER2-positive metastatic breast cancer who have not received prior anti-HER2 therapy or chemotherapy for their metastatic disease.
3.2 The PBAC had not previously considered pertuzumab for listing.
4 Clinical place for the proposed therapy
4.1 In Australia, for more than a decade, trastuzumab in combination with a taxane (docetaxel, paclitaxel or nab-paclitaxel) has become the standard treatment for women with HER2-positive breast cancer. HER2-positive status has been associated with poor prognosis and reduced overall survival. A proportion of patients diagnosed with HER2-positive breast cancer who receive trastuzumab will go on to develop more advanced forms of the disease, and there continues to be a clinical need for additional treatment options for those patients. Examples of lines of treatment for HER2-positive disease are shown in the table below.
|
|
Trastuzumab naïve or sensitive, i.e. relapse from adjuvant treatment >1 year |
Trastuzumab pre-treated and early relapse <1 year (or maybe 6 months) |
|---|---|---|
|
1st line |
Docetaxel + trastuzumab + pertuzumab |
Trastuzumab emtansine |
|
2nd line |
Trastuzumab emtansine |
Lapatinib + capecitabine |
|
3rd line |
Lapatinib + capecitabine |
Lapatinib + trastuzumab a |
|
4th line |
Lapatinib + trastuzumab a |
Trastuzumab + chemotherapy b |
|
5th line |
Trastuzumab + chemotherapy b |
|
a Lapatinib + trastuzumab is not yet an approved treatment combination, but clinical trials (such as EGF 104900) suggest that the combination is effective and therefore would be likely to be used in future practice.
b Chemotherapy = vinorelbine, capecitabine, gemcitabine, carboplatin, any taxane
4.2 Pertuzumab is a first-in-class HER2 dimerisation inhibitor. The combination of pertuzumab, trastuzumab and docetaxel may offer a more comprehensive HER2 blockade. The PBAC considered that the addition of pertuzumab to the PBS would constitute the addition of a line of therapy.
4.3 The PBAC considered that the treatment algorithm for metastatic breast cancer and the cost effectiveness of subsequent therapies would be impacted by both this submission for first-line pertuzumab + trastuzumab combination treatment and the concurrent resubmission for second-line trastuzumab emtansine. The proposed cost-effectiveness of new treatments should be considered in the context of the entire treatment algorithm.
5 Comparator
5.1 The submission nominated trastuzumab + taxane (docetaxel, paclitaxel or nab paclitaxel) as the comparator.
5.2 The PBAC considered this to be the appropriate comparator.
5.3 The PBAC noted that the nominated comparator is not currently listed on the PBS – instead, it is funded under the Herceptin Program. The PBAC recalled that the Herceptin Program was last reviewed by the PBAC in 2008 and that trastuzumab was not found to be acceptably cost effective for PBS listing for patients with HER2-positive metastatic breast cancer. Although the submission offered a price reduction for trastuzumab, the PBAC did not consider that cost-effectiveness of this comparator was yet acceptable based on the evidence provided. The cost effectiveness of pertuzumab depended on the PBAC’s acceptance of the revised cost-effectiveness of trastuzumab.
6 PBAC consideration of the evidence
Consumer comments and sponsor hearing
6.1 The sponsor requested a hearing for this item. The clinician presenting at the hearing spoke in relation to both the pertuzumab and trastuzumab emtansine submissions and noted the enthusiasm amongst clinicians for the listing of these new oncology medicines and discussed the benefits of the new treatments as:
- causing less toxicity (diarrhoea);
- for the few patients who may experience thrombocytopenia side-effects, the clinician considered that these are relatively easy to manage in practice.
6.2 Clarification was sought from the clinician on whether HER2 re-testing is performed in practice. The clinician confirmed that re-testing is performed to ensure that the same cancer (or at least a HER2-positive cancer) is being treated to avoid prescribing the medicine incorrectly.
6.3 Clarification was also sought regarding the price of trastuzumab. The price proposed was the price that would equate to an ICER of $45,000-$75,000/QALY gained for pertuzumab. This would equate to a (REDACTED) price reduction for trastuzumab as confirmed in the pre-PBAC response (p.2) (as opposed to the (REDACTED) price reduction offered in the submission) and accounts for an increase in mark up and dispensing fees paid for by Government if trastuzumab prescribing moved from the Herceptin Program to the PBS.
6.4 The sponsor also confirmed that a sub-cutaneously administered trastuzumab was under development and stated that this was not related to evergreening the patent on trastuzumab.
6.5 The PBAC noted and welcomed the input from individuals (5), health care professionals (12) and organisations (2) via the Consumer Comments facility on the PBS website. The comments described a range of benefits of treatment with pertuzumab including improved survival, increase in quality of life through avoidance of chemotherapy, acceptable tolerability with respect to side effects, and, increased equity in access. The PBAC noted the support for listing received from the Breast Cancer Network Australia and the Medical Oncology Group of Australia (MOGA).
Clinical trials
6.6 The submission was based on one direct (head-to-head), double-blind, placebo-controlled phase III randomised trial (CLEOPATRA) comparing pertuzumab plus trastuzumab plus docetaxel to trastuzumab plus docetaxel in 808 patients with HER2-positive first-line metastatic breast cancer (n=789) or locally recurrent unresectable breast cancer (n=19) who had not received chemotherapy or biologic therapy for their metastatic disease. CLEOPATRA was an ongoing trial with an estimated study completion date of December 2013. Data at two clinical cut-off points were provided (13 May 2011 and 14 May 2012). There was no cross over in CLEOPATRA until the second clinical cut off point (14 May 2012).
6.7 Details of the CLEOPATRA trial are shown in the table below.
|
Trial ID/First Author |
Protocol title/Publication title |
Publication citation |
|---|---|---|
|
CLEOPATRA |
Clinical Study Report – WO20698/TOC4129g: A phase III, randomized, double-blind, placebo-controlled clinical trial to evaluate the efficacy and safety of pertuzumab + trastuzumab + docetaxel vs. placebo + trastuzumab + docetaxel in previously untreated HER2-positive metastatic breast cancer.
|
Research Report No. 1046288. October 2011. |
|
|
Updated Clinical Study Report – WO20698/TOC4129g - A Phase III, Randomized, Double-Blind, Placebo-Controlled Clinical Trial to Evaluate the Efficacy and Safety of Pertuzumab + Trastuzumab + Docetaxel vs. Placebo + Trastuzumab + Docetaxel in Previously Untreated HER2-Positive Metastatic Breast Cancer. |
Report No. 1053649, December 2012. |
|
Swain |
Cardiac tolerability of pertuzumab plus trastuzumab plus docetaxel in patients with HER2-positive metastatic breast cancer in CLEOPATRA: A randomized, double-blind, placebo-controlled phase III study. |
Oncologist 2013; 18(3): 257-264. |
|
Baselga |
Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer.
|
The New England Journal of Medicine 2012; 366(2):109-119.
|
|
Baselga |
CLEOPATRA: a phase III evaluation of pertuzumab and trastuzumab for HER2-positive metastatic breast cancer. |
Clinical Breast Cancer 2010; 10(6):489-491. |
|
Baselga |
Biomarker analysis in CLEOPATRA: a phase III, placebo-controlled study of pertuzumab in HER2-positive, first-line metastatic breast cancer (MBC). |
Cancer Research 2012; 72(24):Suppl.3. Abstract nr S5-1. |
Source: Table B.2.3, p11 of section B of the submission.
Comparative effectiveness
6.8 Overall survival and progression-free survival results from the CLEOPATRA trial are summarised in the table and the Kaplan-Meier curves below.
|
Efficacy parameter |
13 May 2011 cut-off |
14 May 2012 cut-off |
|||||
|---|---|---|---|---|---|---|---|
| Pertuzumab, trastuzumab plus docetaxel N=402 |
Placebo, trastuzumab plus docetaxel N=406 |
Pertuzumab, trastuzumab plus docetaxel N=402 |
Placebo, trastuzumab plus docetaxel N=406 |
||||
|
Overall survival |
|||||||
|
Death |
69 (17.2%) |
96 (23.6%) |
113 (28.1%) |
154 (37.9%) |
|||
|
Absolute risk difference (95% CI) |
6.48% (0.94%, 12.02%) |
9.82% (3.37%, 16.27%) |
|||||
|
Relative risk (95% CI) |
1.085 (1.011, 1.164) |
1.158 (1.051, 1.277) |
|||||
|
Hazard ratio (stratified)** |
0.64 |
0.66 |
|||||
|
95% CI |
0.47, 0.88 |
0.52, 0.84 |
|||||
|
p-value |
0.0053 |
0.0008 |
|||||
|
One year event rate |
|
|
|
|
|||
|
Patients remaining at risk |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
|||
|
Absolute risk difference (95% CI) |
(REDACTED) |
(REDACTED) |
|||||
|
Relative risk (95% CI) |
(REDACTED) |
(REDACTED) |
|||||
|
Event free rate# |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
|||
|
95% CI for rate# |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
|||
|
Independent review facility (IRF) assessed PFS |
|||||||
|
Patients with event* |
(REDACTED) |
(REDACTED) |
Not assessed |
||||
|
Absolute risk difference (95% CI) |
(REDACTED) |
||||||
|
Relative risk (95% CI) |
(REDACTED) |
||||||
|
Hazard ratio |
0.62 |
||||||
|
95% CI |
0.51, 0.75 |
||||||
|
p-value |
<0.0001 |
||||||
|
Investigator-assessed PFS |
|||||||
|
Patients with event |
(REDACTED) |
(REDACTED) |
257 (63.9%) |
296 (72.9%) |
|||
|
Patients without event* |
(REDACTED) |
(REDACTED) |
145 (36.1%) |
110 (27.1%) |
|||
|
Absolute risk difference (95% CI) |
(REDACTED) |
8.98% (2.59%, 15.36%) |
|||||
|
Relative risk (95% CI) |
(REDACTED) |
1.318 (1.073, 1.620) |
|||||
|
Hazard ratio (stratified**) |
0.65 |
0.69 |
|||||
|
95% CI |
0.54, 0.78 |
0.58, 0.81 |
|||||
|
p-value |
<0.0001 |
<0.0001 |
|||||
Sources: Table B.6.2, p60, Table B.6.1, p56 and Table B.6.3, p64 of Section B of the submission.
Notes: compiled during evaluation. Statistically significant results are bolded. Event=IRF-assessed PFS; *Censored; **Stratified by prior treatment status and region; # Kaplan-Meier estimated.
Figure 1: Kaplan-Meier curve of overall survival from the CLEOPATRA trial, data cut-off 14 May 2012 (ITT)
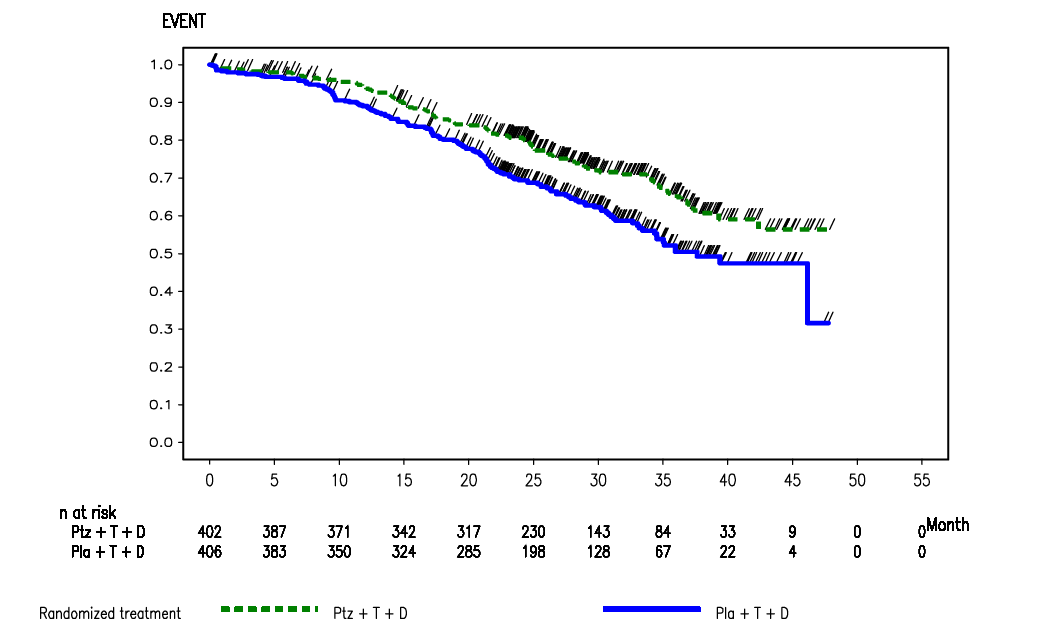
Source: Figure B.6.2, p33 of the commentary.
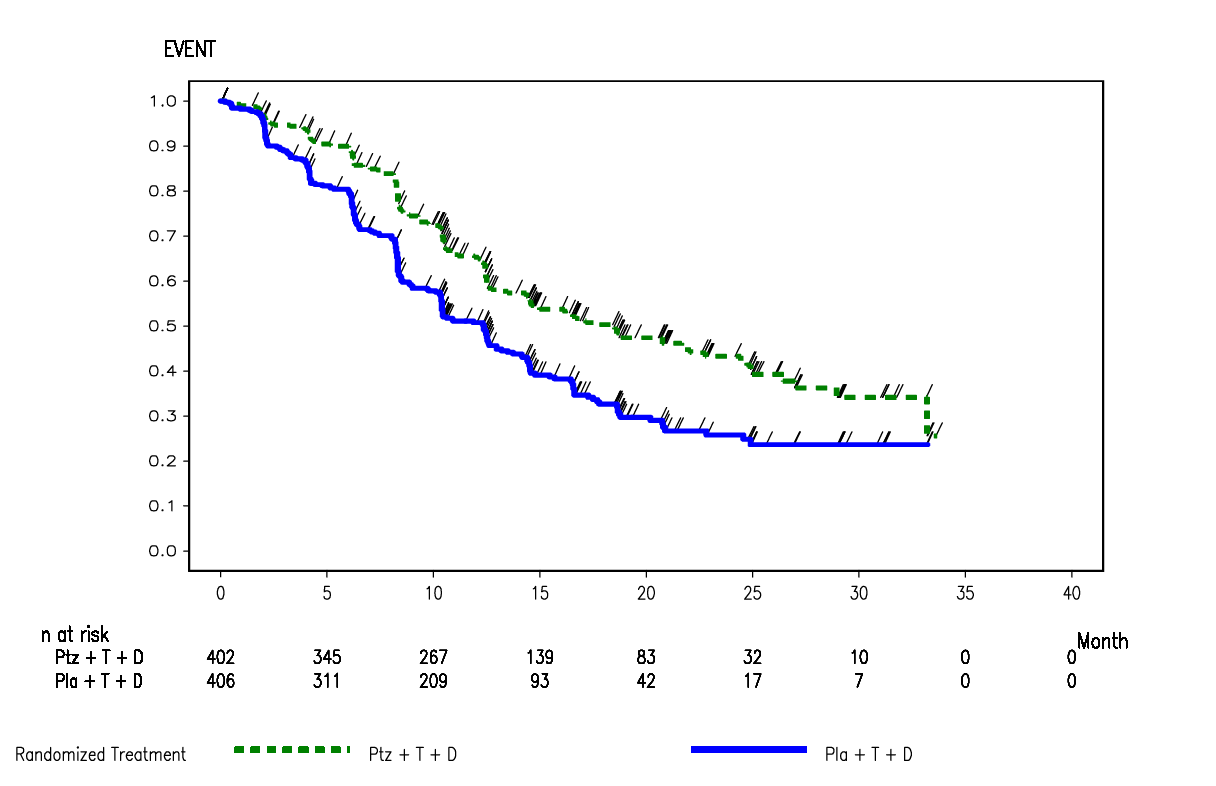
Kaplan-Meier curve of IRF-assessed progression-free survival from the CLEOPATRA trial, data cut-off 13 May 2011 (ITT)
Source: Figure B.6.1, p57 of section B of the submission.
Kaplan-Meier curve of investigator-assessed progression-free survivalfrom the CLEOPATRA trial, data cut-off 14 May 2012 (ITT)
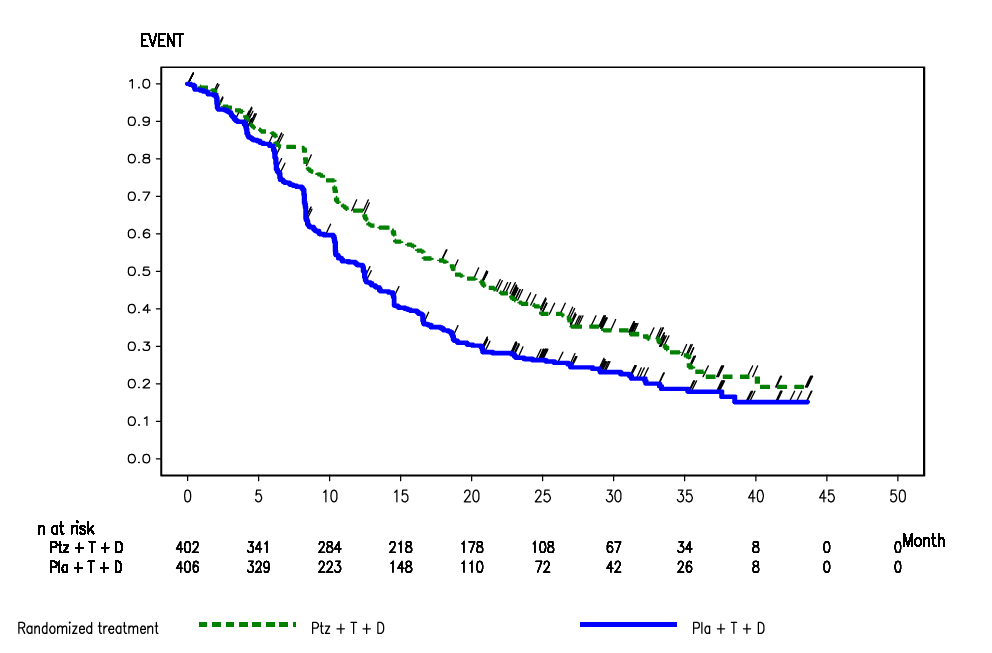
Source: Figure B.6.5, p65 of section B of the submission.
6.9 Overall survival (OS)
At the May 2011 data cut-off, a total of 165 deaths (43% of the pre-specified number for the final analysis) had occurred, with more deaths occurring in the placebo arm compared with the pertuzumab arm (96 deaths versus 69 deaths, respectively). The median time to death had not been reached in either treatment arm. At the May 2012 data cut-off, 267 deaths had occurred with more deaths in the placebo arm (154 deaths; 37.9% of patients) than in the pertuzumab arm (113 deaths; 28.1%).
6.10 At May 2012 data cut-off, the hazard ratio for OS was 0.66 (95%CI: 0.52, 0.84; p=0.0008; stratified by prior treatment status and region) and statistically significant (crosses the pre-defined O’Brien Fleming stopping boundary for the Lan DeMets α-spending function (REDACTED)). The estimated Kaplan-Meier survival rates at 12 months, 24 months and 36 months are 0.94, 0.81 and 0.66 for the pertuzumab arm and 0.89, 0.69 and 0.50 for the placebo arm. Adding pertuzumab statistically significantly increased OS in the CLEOPATRA trial. Median OS had been reached in the placebo arm (37.6 months), but not in the pertuzumab arm. Since the OS data were not mature, it was not possible to estimate the absolute difference in median OS between the pertuzumab arm and the placebo arm. The PBAC noted the clinical benefit of pertuzumab + trastuzumab was greater than trastuzumab.
6.11 Progression-free survival (PFS)
The primary endpoint in the CLEOPATRA trial was Independent Review Facility (IRF)-assessed PFS with the investigator-assessed PFS survival being the secondary endpoints. IRF-assessed PFS was only assessed at the primary analysis (data cut-off 13 May 2011), and then discontinued. Therefore, there were no IRF-assessed PFS data available at the May 2012 data cut.
6.12 At the time of the 13 May 2011 clinical data cut-off, (REDACTED) IRF-assessed PFS events had occurred, (REDACTED) in the placebo arm and (REDACTED) in the pertuzumab arm. IRF-assessed PFS was significantly improved for patients in the pertuzumab arm, compared with patients receiving placebo (HR=0.62, 95% CI: 0.51, 0.75, p<0.0001), with an increase in median PFS of 6.1 months (median 12.4 months for the placebo arm versus 18.5 months for the pertuzumab arm). At the time of the 14 May 2012 clinical data cut-off, 553 patients (68%) were reported to have had a PFS event according to investigator assessment. More patients in the placebo arm (296 patients, 72.9%) had a PFS event compared with patients treated with pertuzumab (257 patients, 63.9%). The HR was 0.69 (95% CI: 0.58, 0.81), and the increase in median PFS was 6.3 months, from 12.4 months in the placebo arm to 18.7 months in the pertuzumab arm. Adding pertuzumab statistically significantly increased PFS in the CLEOPATRA trial.
6.13 The submission claimed that given the overall agreement between IRF and investigator (defined as agreement on an event occurring or agreement on no event occurring) being approximately 85% in both treatment arms, concordance between the IRF and investigators was high.
6.14 The submission also presented estimates of objective response rate, duration of objective response and time to symptom progression:
- The results of the IRF-assessed objective response suggested that a higher percentage of patients in the pertuzumab arm achieved an objective response, compared with those on the comparator arm (80.2% versus 69.3%, respectively, p=0.0011, stratified analysis). The difference in response rates was 10.83% (95% CI: 4.34%, 17.32%).
- The median duration of response in the comparator arm was 54.1 weeks versus 87.6 weeks in the pertuzumab arm (HR=0.66, 95% CI: 0.51, 0.85), based on IRF-assessed data.
- The results showed no evidence that the addition of pertuzumab to trastuzumab and docetaxel led to a detrimental effect on quality of life, with 229 patients (56.7%) in the comparator treatment arm and 239 patients (59.5%) in the pertuzumab treatment arm experiencing symptom progression. The median time to deterioration was 18.3 weeks versus 18.4 weeks, representing approximately six cycles, with a hazard ratio of 0.97 (95% CI: 0.81,1.16).
6.15 The PBAC considered that based on the trial evidence presented, the effect of pertuzumab in combination with docetaxel and trastuzumab is clinically significant in trastuzumab naïve (sensitive) patients.
Comparative harms
6.16 In the CLEOPATRA trial, the incidence of serious adverse events (SAEs) and adverse events (AEs) were both statistically significantly higher in the pertuzumab arm compared with the placebo arm. Also, there was a statistically significantly higher incidence of AEs leading to dose modifications or interruptions in the pertuzumab arm. Pertuzumab was associated with more cases of grade 1 or 2 diarrhoea, rash, mucosal inflammation, dry skin, and grade 3 or 4 febrile neutropenia. The incidence of treatment-related SAEs was higher in the pertuzumab arm (REDACTED) compared with the placebo arm (REDACTED). The majority of these AEs were blood and lymphatic system disorders (REDACTED) of patients in the pertuzumab arm and (REDACTED) of patients in the placebo arm). In particular, the incidence of serious treatment-related febrile neutropenia occurred statistically significantly more frequently in the pertuzumab arm (REDACTED) compared with the placebo arm (REDACTED).
6.17 The PBAC noted that some SAEs and AEs were higher in the pertuzumab arm compared to the placebo arm. The safety profile in both arms was dominated by events typically associated with docetaxel treatment. Overall, the PBAC noted there was no unexpected safety finding and no detrimental effect on cardiac safety.
6.18 The PBAC considered the trial results indicated that adding pertuzumab to trastuzumab and docetaxel results in statistically significant increased toxicity in trastuzumab naïve (sensitive) compared to trastuzumab in combination with docetaxel.
6.19 A summary of the comparative benefits and harms for pertuzumab (+trastuzumab and docetaxel) versus placebo (+trastuzumab and docetaxel) is presented in the table below.
|
Outcome |
N |
RR (95% CI) |
Median months (95% CI) |
Increment |
|
|---|---|---|---|---|---|
| Pertuzumab, trastuzumab plus docetaxel | Placebo, trastuzumab plus docetaxel | ||||
|
Benefits |
|||||
|
PFS (May 2012 cut-off) |
808 |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
|
OS (May 2012 cut-off) |
808 |
1.158 (1.051, 1.277) |
Not reached |
37.6 |
N/A |
|
Harms |
Proportions |
|
|||
|
All grade ³3 AEs |
804 |
1.037 (0.957, 1.124) |
76.2% |
73.5% |
+2.7% |
|
Grade ³3 febrile neutropenia |
804 |
1.874 (1.223, 2.871) |
13.7% |
7.3% |
5.4% |
|
Grade ³3 diarrhoea |
804 |
1.872 (0.996, 3.516) |
6.6% |
3.5% |
3.1% |
Source: compiled during the evaluation
6.20 The PBAC noted that the latest analysis of the data from the CLEOPATRA trial, which is the report from May 2012, showed that after approximately 4 years of treatment, approximately 28% of patients treated with the combination including pertuzumab had died, compared with approximately 38% in the group not treated with pertuzumab. In other words, the addition of pertuzumab to the treatment regimen of 100 people would result in 72 people being alive at 4 years, rather than 62.
6.21 As well for every 100 patients treated with pertuzumab (+trastuzumab and docetaxel) compared to just trastuzumab in combination with docetaxel:
- approximately 3 more patients would experience a serious adverse event;
- approximately 5 more patients would experience the side-effect of grade 3 or higher febrile neutropenia; and
- approximately 3 more patients would experience the side-effect of grade 3 or higher diarrhoea.
Clinical claim
6.22 The submission described pertuzumab, when used in combination with trastuzumab plus docetaxel, as superior in terms of comparative effectiveness and “slightly worse” in terms of comparative safety over trastuzumab plus docetaxel alone.
6.23 The PBAC considered this clinical claim to be reasonable.
Economic analysis
6.24 The submission presented a modelled economic evaluation (cost-utility analysis) based on the claim of superior efficacy. The submission calculated an ICER of $45,000-$75,000/QALY based on estimates for PFS and OS from CLEOPATRA, and extrapolated to ten years duration (from 29.9 months follow-up in the trial) and then applying utility weights from Lloyd (2006) (except for the disease progressed state). A proposed risk share agreement (REDACTED) was also included in the model.
6.25 The cost-effectiveness of pertuzumab depended on the cost-effectiveness of its comparator, trastuzumab + taxane in HER2-positive metastatic breast cancer. As there was also an offer of a (REDACTED) price reduction (REDACTED) for this use and any subsequent use of trastuzumab, the PBAC considered that the essential prerequisite to considering pertuzumab was to determine whether trastuzumab would now be sufficiently cost-effective to recommend the PBS listing of trastuzumab to include all the use of trastuzumab currently funded via the Herceptin Program and thus recommend the end of the Herceptin Program.
6.26 The ICER of trastuzumab + taxane, after a (REDACTED) price reduction, was estimated to be in the range of $45,000-$75,000/QALY over chemotherapy. It was noted that a combination of the price reduction and the RPSFT method of adjusting for cross over in the trial were the main drivers of the reduction in the ICER, compared to the $105,000 to $200,000/QALYgenerated by the corresponding AUC model in 2008. The base case was therefore a likely underestimate and the ensuing ICER of HER2 blockade (pertuzumab + trastuzumab + docetaxel) compared to trastuzumab + taxane alone was also likely to be underestimated. A sensitivity analysis of the AUC model for trastuzumab + docetaxel compared to docetaxel treatment alone that was provided in the Commentary generated an ICER in the range of $45,000 to $75,000/QALY (5.12 COM.99). The pre-sub-committee response (p.2) stated that ‘Multivariate sensitivity analyses including 5% discounting, CT scans and log-logistic extrapolation of OS yield ICERs in the range of $45,000 - $75,000 (same hazard of death after truncation point( or different hazard of death after truncation point)’. The model included in the submission was unable to be used to verify the new information presented in the PSCR.
6.27 The PBAC considered an update of the 2008 economic model assessing the cost-effectiveness of trastuzumab + taxane is appropriate in order to address the issues raised in 2008, the availability of a 60 mg vial and changes in unit costs (e.g. prices of the comparator medicines, dispensing fees and mark-ups etc.). However, the PBAC agreed with the ESC that there were substantial issues with the corresponding economic evaluation presented in the current submission. These were:
- It is unknown exactly when patients crossed-over; no consideration was given to the appropriateness of applying the rank preserving structural failure time (RPSFT) method versus the inverse probability of censoring weighting (IPCW) method; and the results of the adjustment for cross-over were unable to be verified independently. The directions of bias were unknown, but potentially may have favoured trastuzumab. The ESC advised that the RPSFT method is appropriate if the data is normally distributed and that the pre-sub-committee response (p.1) discussed why the IPCW method was inappropriate to apply to the data. However, the PBAC noted that a sensitivity analysis of using the RPSFT method rather than the IPCW method indicated that there was limited variation in the ICER. The PBAC considered that the greater issue was the overall acceptability of either adjustment for cross-over.
- The goodness of fit of the exponential function used to extrapolate PFS and OS was poor. The direction of bias was unknown, but potentially may have favoured trastuzumab. The pre-sub-committee response (p.1, Table 1) presented a sensitivity analysis ‘using different parametric functions fitted to the cross-over adjusted OS data yield lower ICERs compared with the base case, both if the same hazard of death is applied or a sustained difference in OS is assumed’. The PBAC noted that there was limited variation in the ICER according to the methods used.
- There was no discounting applied in the model. This is likely to have favoured trastuzumab. The ESC advised that this was inappropriate in the context of the PBAC Guidelines. The pre-sub-committee response (p.2) stated that ‘Applying a 5% discount rate per annum increases the base-case ICER to between $45,000 and $75,000 per QALYG’.
- The utility value for “progressing disease” (0.7) is likely to have been over-estimated. The current estimate favours trastuzumab. The PBAC noted that the economic analysis of trastuzumab and pertuzumab/ trastuzumab both applied a utility value of 0.7 for the progressive state of metastatic breast cancer, while alternate values such as 0.55 are published. The pre-sub-committee response (p.2) stated that ‘The sponsor maintains that the utility value of 0.7 for progressing disease following first-line MBC…is appropriate’. However, the ESC advised that, in this submission, the choice of utilities has low impact on the ICER.
- The cost of CT scans was not included. This is likely to have favoured trastuzumab given the longer PFS and OS (and thus number of scans). The pre-sub-committee response (p.2) stated that the ‘correct ICER including 12-weekly CT scans in both arms is within the range of $45,000 - $75,000 but this reanalysis did not include appropriate discounting.
- Disutilities and costs due to adverse events were not included. This is likely to have favoured trastuzumab. The pre-sub-committee response stated that ‘Costs and disutilities for the management of adverse events were excluded for consistency with the 2008 model. This is reasonable because the main adverse events experienced by patients in the M77001 trial were due to taxane chemotherapy and are similar in both arms.’
6.28 The PBAC also expressed concern that this model for trastuzumab should reflect the incremental costs and effectiveness over chemotherapy and best supportive care of contemporary long-term trastuzumab use (both in combination with other partner therapies and as monotherapy) until death. Costs and outcomes are assumed to only diminish due to discounting, whereas the PBAC expected that there would be likely diminishing marginal returns in terms of the effectiveness of trastuzumab over time, especially when given as monotherapy. The usage patterns of trastuzumab confirm this practice of continued use; despite the advice from clinical experts in 2008 that trastuzumab would be commenced in the second-line management of metastatic breast cancer only in rare circumstances. These patterns are also supported by the 2011 increase in PBS use of lapatinib when its restriction was changed to allow patients to return to Herceptin program funded trastuzumab after lapatinib had failed. In clinical practice, trastuzumab is usually combined with a range of cytotoxics and/or hormonal therapy. In estimating incremental costs and outcomes of HER2 blockade for metastatic breast cancer it is important that the entire treatment algorithm is considered. This economic evaluation should take into account the timing of initial and subsequent use of trastuzumab as well as its use with various partners and as monotherapy.
6.29 As noted earlier under ‘Clinical place for the proposed therapy’, the PBAC considered that the proposed cost-effectiveness of new treatments in HER2-positive metastatic breast cancer should be considered in the context of the entire treatment algorithm.
6.30 A particular example of the importance of considering the entire treatment algorithm is the implausibility that, despite the model for pertuzumab estimating that the post progression state (REDACTED) accounts for approximately 30% of the incremental survival gain with pertuzumab (REDACTED) the economic model assumed that this health state either contributed no costs (base case) or cost off-sets (sensitivity analysis). Issues with this aspect of the model included:
- the value assigned to post-progression costs when included: costs were assumed to be the same per cycle for both arms (for lapatinib and capecitabine), but the net present value per cycle was less for the pertuzumab arm because these costs occur later on average and so were differentially affected by discounting. Whether this discounting effect is sufficient to outweigh the estimated longer duration in the post-progression state was not clear;
- when included, post-progression costs were for only one extra line of therapy, which is not consistent with Australian practice to move on to later lines of therapy (for example the recent change in the PBS restriction for lapatinib to allow patients to return to trastuzumab-based therapy), but if it is reasonable to assume the extra lines of therapy would be the same, this is likely to be just an extension of the first issue;
- post-progression trastuzumab is not included, so, as constructed, the ICER was not affected by the (REDACTED) price reduction for trastuzumab offered in the submission;
- the submission did not provide data from the CLEOPATRA trial on the proportions of patients receiving post-progression therapy after pertuzumab or its comparator.
6.31 The ESC advised that failing to account adequately for extra post-progression costs with pertuzumab compared with its comparator inappropriately affected the estimated ICER.
6.32 The PBAC noted that, in the consideration of pertuzumab’s cost-effectiveness, the base case ICER was claimed to be in the range of $45,000 - $75,000/QALY and could realistically vary from between $75,000/QALY to $105,000/QALY (assuming parametric extrapolation of PFS using Weibull function) to between $45,000/QALY and $75,000/QALY (assuming price disclosure-related reductions in the price of docetaxel and paclitaxel, as per the pre-sub-committee response, p.5). The PBAC considered that the ICER (pertuzumab + trastuzumab + docetaxel versus trastuzumab + docetaxel) was highly sensitive to the risk-sharing arrangement (REDACTED). The PBAC did not consider that it would be feasible to implement such a risk-sharing arrangement, (REDACTED). Without the (REDACTED) risk-sharing arrangement, the ICER increases to above $200,000/QALY. (REDACTED).
6.33 Overall, the PBAC considered the pertuzumab economic model to be reasonably reliable despite the issues outlined above and the potential for the ICER to be underestimated. However, in the absence of confidence in the cost-effectiveness of trastuzumab in HER2-positive metastatic breast cancer, the submission’s claimed ICER for pertuzumab + trastuzumab + docetaxel versus trastuzumab + docetaxel in the range of $45,000 - $75,000 was still considered by the PBAC to be unacceptably high. A positive PBAC recommendation to list pertuzumab would depend on the PBAC being sufficiently satisfied that listing trastuzumab on the PBS for HER2-positive metastatic breast cancer (the comparator) is acceptably cost-effective. This was not yet the case. In view of the improved survival benefit that pertuzumab has to offer, the PBAC recommended the Department engage with the sponsor, professional advocating oncology groups and the sponsor of lapatinib, in an endeavour to progress the potential updated PBS listings of all four HER2 blocking medicines: trastuzumab, pertuzumab, trastuzumab emtansine and lapatinib.
Estimated PBS usage & financial implications
6.34 This submission was not considered by DUSC.
6.35 The likely number of patients per year was estimated in the submission to be less than 10,000 per year. The net cost to PBS was estimated to be between $250 million and $300 million over 5 years with savings to the Herceptin Program between $150 million and $200 million over 5 years.
|
|
Year 1 |
Year 2 |
Year 3 |
Year 4 |
Year 5 |
|---|---|---|---|---|---|
|
Estimated extent of use |
|||||
|
Number treated |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
|
Total pertuzumab prescriptions (cycles) written per year |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
|
Estimated net cost to PBS/RPBS/MBS |
|||||
|
Overall net cost to PBS (patient co-payments removed) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
|
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
|
|
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
|
|
Overall net cost to MBS (patient co-payments removed) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
|
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
|
|
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
|
|
Cost offset for Herceptin Program |
|||||
|
Cost offset for Herceptin Program |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
|
Estimated total net cost |
|||||
|
Overall net cost to government health budget |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
(REDACTED) |
Source: Table E.5.5, p84 of the Commentary.
(REDACTED)
6.36 The PBAC considered that the overall net cost to Government as presented in the table above was lower than expected and not consistent with what would be anticipated from additional pertuzumab drug costs minus cost savings arising from a trastuzumab price reduction. The PBAC noted that, regardless of how trastuzumab is subsidised (i.e. through the Herceptin Program or via the PBS), if current annual Government expenditure on trastuzumab in metastatic breast cancer is approximately $60 million, a (REDACTED) price reduction to the current price of trastuzumab would mean that the offset to Government annual expenditure on trastuzumab should be between approximately $10 million and $30 million. This is less than the projections for the component of use funded via the Herceptin program. Given that pertuzumab is expected to increase life expectancy by approximately 1 year, current trastuzumab drug treatment costs would be further expected to increase by approximately (REDACTED) treatment cycles (REDACTED)'. As the PBAC considered that the combination of pertuzumab + trastuzumab + docetaxel would effectively be adding another line of therapy, accounting for additional lapatinib costs (and potentially trastuzumab emtansine costs) would make the overall net costs to Government as presented in the above table appear to be even greater underestimates.
6.37 The PBAC therefore considered that, if pertuzumab was to be recommended for listing, the Department should re-examine the projected financial implications considering the following factors:
- the expected rate of pertuzumab uptake, given that the Herceptin Program took approximately 4 years to reach a plateau in the number of patients receiving trastuzumab;
- an increase in annual incidence of metastatic breast cancer of approximately 2.3%;
- the duration of therapy on each drug (REDACTED);
- the expectation that HER2 blockade is likely to continue beyond progression in practice;
- the average (including 95% CI) duration of exposure to trastuzumab on the Herceptin Program, in patient categories according to use of cytotoxics and/or hormonal therapies. This includes, the number of switches of cytotoxics whilst maintaining backbone trastuzumab and the impact of these switches on the duration of exposure to trastuzumab. Also of interest is the duration and timing of monotherapy trastuzumab and in particular whether patients only receive monotherapy trastuzumab after failure of combination therapy, or they receive monotherapy as a “drug holiday” between combination treatments.
Based on these factors, the following estimates could be established:
- the number of prevalent patients on pertuzumab at steady state;
- the time taken to reach a steady state in terms of the number of patients treated with pertuzumab; and
- the average duration of exposure to each HER2 blocking medicine over the life time of a patient.
6.38 The PBAC noted that the submission proposed a risk-sharing arrangement. (REDACTED). A (REDACTED) price reduction was also proposed on the ex-manufacturer price of trastuzumab when used for the treatment of metastatic breast cancer. It was the PBAC’s understanding that the risk-sharing arrangement only applied when pertuzumab and trastuzumab were used in combination in HER2-positive first-line metastatic breast cancer. The PBAC noted that if the risk-sharing arrangement could not be administered, the ICER increased from between $45,000 and $75,000/QALY to above $200,000/QALY. (REDACTED) the PBAC did not consider (REDACTED) the proposed risk-sharing arrangement was reasonable.
6.39 The PBAC further postulated that, in practice, what might happen is that pertuzumab + trastuzumab would be used together initially and then pertuzumab treatment would be ceased, as well as docetaxel, if progression occurs, noting that pertuzumab may increase the occurrence of side effects. In clinical practice, the incentive would be to reduce toxicity to maintain long-term treatment with trastuzumab if progression occurs. The proposed risk-sharing arrangement would not appear to protect against this scenario. In relation to the practicality of implementing such an arrangement, it was unclear to the PBAC how breaks in therapy would be dealt with (REDACTED).
6.40 In light of the perceived difficulties in administering the proposed risk sharing arrangement (REDACTED), at this stage, the PBAC was of the view that a risk sharing arrangement based on an expected annual expenditure on each HER2 blocking medicine with a 100% rebate beyond each cap, to be the most acceptable to manage unexpected Government expenditure on HER2 blocking medicines.
7 PBAC Outcome
7.1 The PBAC deferred making a recommendation on listing pertuzumab to enable it to first consider, establish and accept the cost-effectiveness of the comparator (trastuzumab + docetaxel) as first-line treatment of metastatic breast cancer, before making a judgement on the cost-effectiveness of all HER2 blocking medicines in metastatic breast cancer, including pertuzumab.
Outcome:
Deferred
8 Sponsor comment
Roche is disappointed by the decision of the PBAC not to recommend PERJETA for inclusion on the PBS. Roche has carefully considered the PBAC’s commentary and is working with the Committee and stakeholders to address outstanding issues of concern such that this much needed medicine can be PBS listed at the earliest possible opportunity.




