Price Disclosure Reductions for 2014 October Cycle
(First cycle under Simplified Price Disclosure amendments)
The price disclosure outcomes for this cycle were calculated in accordance with the National Health Act 1953 (the Act) and National Health (Pharmaceutical Benefits) Regulations 1960 (the Regulations) as amended for Simplified Price Disclosure.
To assist readers to understand the 2014 October Cycle Outcomes Summary, the diagram sets out the processes and sample inputs for the regular cycle with a 1 October 2014 reduction day. It shows the two types of price differences that are important under the Simplified Price Disclosure amendments (see blue/ vertical and red/ diagonal arrows - further explanation below). The PBS Price is the base price before mark-ups and fees. A PDF version of the Diagram (146 KB) is also available.
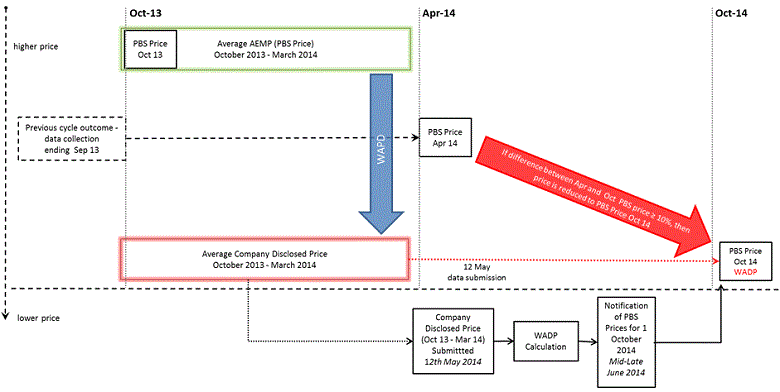
In the Outcomes Summary below:
MoA = manner of administration of a form of drug
Average AEMP = the weighted average approved ex-manufacturer price (PBS price) for brands of the pharmaceutical item during the data collection period ending 31 March 2014. Average AEMP is calculated in accordance with Regulation 37J (Step 3) of the calculation method in the Regulations. *
WAPD (drug/MoA) = the weighted average percentage difference between the Average AEMPs and the prices disclosed by companies across all brands of a drug and manner of administration (drug/MoA). This is the blue/vertical arrow in the diagram above. WAPD is calculated in accordance with Regulation 37R (Step 10) of the Regulations. *
WADP (pharmaceutical item) = the weighted average disclosed price, which is worked out by reducing the Average AEMP for a brand by its WAPD. See Regulation 37S (Step 11) of the Regulations. This is the amount determined in the price disclosure legal determination for each brand of pharmaceutical item. For medicines that meet the 10% test, this amount is also determined as the adjusted approved ex-manufacturer price, which will become the new base PBS price, before fees and mark-ups, on 1 October 2014 (unless there is an intervening lower price). *
April AEMP = the approved ex-manufacturer price effective on 1 April 2014 for the pricing quantity for brands of the relevant pharmaceutical item. The April 2014 price may be different to the Average AEMP during the data collection period, for example, due to a 1 April 2014 price disclosure reduction or PBPA price increase, or to a price change during the data collection period.
April to October 2014 Price Difference = the percentage difference between:
- the 1 April 2014 approved ex-manufacturer price for brands of each pharmaceutical item; and
- the proposed 1 October 2014 new base PBS price. This price is equivalent to the WADP (i.e.: Average AEMP minus WAPD). The actual 1 October 2014 base PBS price may change if there are changes in prices or listings before then (e.g.: lower price / pricing quantity changes).
The April to October price difference is the red/ diagonal arrow in the diagram above. This percentage difference is the one used for the 10% test under subsection 99ADH(1)(c) of the Act.* Only medicines with at least a 10% difference between the price on the relevant day (1 April 2014 in this case) and the WADP meet the 10% test for a 1 October 2014 reduction. All the medicines in the Outcome Summary below meet the 10% test for reduction.
The April to October percentage difference may be different across different pharmaceutical items of the same drug/MoA because of, for example:
- rounding when applying the WAPD to different pharmaceutical item prices; or
- different price increases on the relevant day (1 April) for different pharmaceutical items; or
- differences in the weighting of the different Average AEMPs for different items because of a price change or item listing at different times in the cycle.
*Further explanation of the concepts above, and the calculation for price disclosure, is included in the explanation of the price disclosure method and sample price disclosure calculation on the Price Disclosure (SPD) web page.
Legal Determination & Next Steps
The weighted average disclosed prices for each brand, including brands with no reduction, are in the legal determination on the ComLaw website. Price disclosure reductions will apply to brands listed in Schedule 1 of the legal determination. Those in Schedule 2 of the determination will not take a price disclosure reduction because the percentage difference between their April PBS approved ex-manufacturer price and the weighted average disclosed price was below 10%. A new brand listed after 31 March 2014 for a pharmaceutical item that has an existing brand with a determination in Schedule 1 of the determination will also take the reduction on 1 October 2014 under section 99ADHA of the Act.
Final prices applicable on 1 October 2014 (including price to pharmacy, Dispensed Price Maximum Quantity and price premiums) will be available under a ‘Confirmation of New Prices Resulting from Price Disclosure Cycles’ link on this web page from early September 2014. To further assist companies and other stakeholders indicative 1 October 2014 prices (including price to pharmacy, Dispensed Price Maximum Quantity and price premiums) will be published by mid-July 2014, after completion of further pricing calculations. Subscribe to PBS News to receive notice when they become available.
An agreed process for responsible persons to raise any questions or concerns arising from an outcome mentioned above is set out in the Price Disclosure Dispute Resolution Administrative Process. The date for lodgement of disputes is 5 pm AEST on Thursday, 26 June 2014.
Note: The documents on this page may not be accessible. If you need assistance please email the Price Disclosure team.
2014 October Cycle Outcomes Summary
This summary only covers medicines taking a reduction. The price disclosure legal determination mentioned above includes brands with no reductions.
NOTE: The prices below do not include fees and mark-ups. Indicative new 1 October 2014 prices, with fees and mark-ups, will be published by mid-July 2014.
Outcomes for brands of pharmaceutical items containing filgrastim for injection in the 2014 October Cycle have been amended. Data was resubmitted for these brands following consideration of matters raised by a responsible person.
| Legal Instrument Drug | Legal Instrument MoA | Legal Instrument Form | Average AEMP ($) | WAPD (%) across a drug/MoA |
WADP potential October 14 PBS Price (AEMP) ($) per pharmaceutical item |
April 14 AEMP ($) |
Percentage difference between April 14 and October 14 PBS prices (AEMP) (%) per pharmaceutical item |
|---|---|---|---|---|---|---|---|
| [Average AEMP minus WAPD = WADP] |
[Difference between April AEMP & WADP = % Difference] |
||||||
| Aciclovir | Oral | Tablet 200 mg | 16.05 | 35.35% | 10.38 | 11.97 | 13.28% |
| Aciclovir | Oral | Tablet 800 mg | 69.90 | 35.35% | 45.19 | 52.11 | 13.28% |
| Alendronic acid | Oral | Tablet 40 mg (as alendronate sodium) | 38.41 | 43.22% | 21.81 | 27.65 | 21.12% |
| Alendronic acid | Oral | Tablet 70 mg (as alendronate sodium) | 11.63 | 43.22% | 6.60 | 8.37 | 21.15% |
| Allopurinol | Oral | Tablet 100 mg | 2.14 | 14.10% | 1.84 | 2.14 | 14.02% |
| Allopurinol | Oral | Tablet 300 mg | 2.52 | 14.10% | 2.16 | 2.52 | 14.29% |
| Alprazolam | Oral | Tablet 1 mg | 4.89 | 12.63% | 4.27 | 4.89 | 12.68% |
| Alprazolam | Oral | Tablet 2 mg | 7.58 | 12.63% | 6.62 | 7.58 | 12.66% |
| Alprazolam | Oral | Tablet 250 micrograms | 1.74 | 12.63% | 1.52 | 1.74 | 12.64% |
| Alprazolam | Oral | Tablet 500 micrograms | 2.81 | 12.63% | 2.46 | 2.81 | 12.46% |
| Amisulpride | Oral | Tablet 100 mg | 17.33 | 32.23% | 11.74 | 13.36 | 12.13% |
| Amisulpride | Oral | Tablet 200 mg | 74.64 | 32.23% | 50.58 | 57.53 | 12.08% |
| Amisulpride | Oral | Tablet 400 mg | 135.35 | 32.23% | 91.73 | 104.33 | 12.08% |
| Amlodipine | Oral | Tablet 10 mg (as besylate) | 4.28 | 40.69% | 2.54 | 2.94 | 13.61% |
| Amlodipine | Oral | Tablet 5 mg (as besylate) | 2.44 | 40.69% | 1.45 | 1.68 | 13.69% |
| Ampicillin | Injection | Powder for injection 1 g (as sodium) | 5.88 | 11.04% | 5.23 | 5.88 | 11.05% |
| Ampicillin | Injection | Powder for injection 500 mg (as sodium) | 3.58 | 11.04% | 3.18 | 3.58 | 11.17% |
| Anastrozole | Oral | Tablet 1 mg | 110.72 | 30.91% | 76.50 | 91.69 | 16.57% |
| Atorvastatin | Oral | Tablet 10 mg (as calcium) | 14.89 | 66.23% | 5.03 | 9.30 | 45.91% |
| Atorvastatin | Oral | Tablet 20 mg (as calcium) | 21.98 | 66.23% | 7.42 | 13.72 | 45.92% |
| Atorvastatin | Oral | Tablet 40 mg (as calcium) | 30.94 | 66.23% | 10.45 | 19.32 | 45.91% |
| Atorvastatin | Oral | Tablet 80 mg (as calcium) | 44.23 | 66.23% | 14.94 | 27.62 | 45.91% |
| Azathioprine | Oral | Tablet 25 mg | 18.09 | 13.01% | 15.74 | 18.09 | 12.99% |
| Azathioprine | Oral | Tablet 50 mg | 30.80 | 13.01% | 26.79 | 30.80 | 13.02% |
| Azithromycin | Oral | Tablet 500 mg (as dihydrate) | 11.87 | 49.50% | 5.99 | 7.68 | 22.01% |
| Azithromycin | Oral | Tablet 600 mg (as dihydrate) | 61.68 | 49.50% | 31.15 | 36.89 | 15.56% |
| Baclofen | Oral | Tablet 10 mg | 12.15 | 14.59% | 10.38 | 12.15 | 14.57% |
| Baclofen | Oral | Tablet 25 mg | 26.56 | 14.59% | 22.68 | 26.56 | 14.61% |
| Bicalutamide | Oral | Tablet 50 mg | 122.79 | 22.66% | 94.97 | 105.62 | 10.08% |
| Bisoprolol | Oral | Tablet containing bisoprolol fumarate 10 mg | 44.24 | 41.53% | 25.87 | 32.87 | 21.30% |
| Bisoprolol | Oral | Tablet containing bisoprolol fumarate 2.5 mg | 28.32 | 41.53% | 16.56 | 21.04 | 21.29% |
| Bisoprolol | Oral | Tablet containing bisoprolol fumarate 5 mg | 35.39 | 41.53% | 20.69 | 26.29 | 21.30% |
| Calcitriol | Oral | Capsule 0.25 microgram | 22.27 | 13.33% | 19.30 | 22.27 | 13.34% |
| Candesartan | Oral | Tablet containing candesartan cilexetil 16 mg | 15.28 | 17.92% | 12.54 | 15.28 | 17.93% |
| Candesartan | Oral | Tablet containing candesartan cilexetil 32 mg | 18.47 | 17.92% | 15.16 | 18.47 | 17.92% |
| Candesartan | Oral | Tablet containing candesartan cilexetil 4 mg | 1.71 | 17.92% | 1.40 | 1.71 | 18.13% |
| Candesartan | Oral | Tablet containing candesartan cilexetil 8 mg | 5.11 | 17.92% | 4.19 | 5.11 | 18.00% |
| Candesartan with hydrochlorothiazide | Oral | Tablet containing candesartan cilexetil 16 mg with hydrochlorothiazide 12.5 mg | 16.79 | 22.58% | 13.00 | 16.79 | 22.57% |
| Candesartan with hydrochlorothiazide | Oral | Tablet containing candesartan cilexetil 32 mg with hydrochlorothiazide 12.5 mg | 19.98 | 22.58% | 15.47 | 19.98 | 22.57% |
| Candesartan with hydrochlorothiazide | Oral | Tablet containing candesartan cilexetil 32 mg with hydrochlorothiazide 25 mg | 21.49 | 22.58% | 16.64 | 21.49 | 22.57% |
| Captopril | Oral | Tablet 12.5 mg | 5.02 | 14.36% | 4.30 | 5.02 | 14.34% |
| Captopril | Oral | Tablet 25 mg | 7.26 | 14.36% | 6.22 | 7.26 | 14.33% |
| Captopril | Oral | Tablet 50 mg | 14.33 | 14.36% | 12.27 | 14.33 | 14.38% |
| Carboplatin | Injection | Solution for I.V. injection 150 mg in 15 mL | 15.48 | 13.24% | 13.43 | 15.48 | 13.24% |
| Carboplatin | Injection | Solution for I.V. injection 450 mg in 45 mL | 29.16 | 13.24% | 25.30 | 29.16 | 13.24% |
| Carboplatin | Injection | Solution for I.V. injection 50 mg in 5 mL | 6.41 | 13.24% | 5.56 | 6.41 | 13.26% |
| Cefalotin | Injection | Powder for injection 1 g (as sodium) | 13.31 | 23.67% | 10.16 | 11.35 | 10.48% |
| Cefepime | Injection | Powder for injection 1 g (as hydrochloride) | 8.74 | 47.85% | 4.56 | 6.95 | 34.39% |
| Cefepime | Injection | Powder for injection 2 g (as hydrochloride) | 16.65 | 47.85% | 8.68 | 13.25 | 34.49% |
| Cefotaxime | Injection | Powder for injection 1 g (as sodium) | 1.33 | 10.38% | 1.19 | 1.33 | 10.53% |
| Cefotaxime | Injection | Powder for injection 2 g (as sodium) | 2.45 | 10.38% | 2.20 | 2.45 | 10.20% |
| Ceftriaxone | Injection | Powder for injection 1 g (as sodium) | 2.41 | 43.61% | 1.36 | 1.55 | 12.26% |
| Ceftriaxone | Injection | Powder for injection 2 g (as sodium) | 4.47 | 43.61% | 2.52 | 2.87 | 12.20% |
| Ceftriaxone | Injection | Powder for injection 500 mg (as sodium) | 1.54 | 43.61% | 0.87 | 0.99 | 12.12% |
| Cephazolin | Injection | Powder for injection 1 g (as sodium) | 7.93 | 56.57% | 3.44 | 4.15 | 17.11% |
| Cephazolin | Injection | Powder for injection 2 g (as sodium) | 3.64 | 56.57% | 1.58 | 1.90 | 16.84% |
| Cephazolin | Injection | Powder for injection 500 mg (as sodium) | 5.96 | 56.57% | 2.59 | 3.12 | 16.99% |
| Ciprofloxacin | Oral | Tablet 250 mg (as hydrochloride) | 6.54 | 32.57% | 4.41 | 5.12 | 13.87% |
| Ciprofloxacin | Oral | Tablet 500 mg (as hydrochloride) | 12.79 | 32.57% | 8.62 | 10.01 | 13.89% |
| Ciprofloxacin | Oral | Tablet 750 mg (as hydrochloride) | 19.28 | 32.57% | 13.00 | 15.09 | 13.85% |
| Citalopram | Oral | Tablet 10 mg (as hydrobromide) | 2.35 | 43.75% | 1.32 | 1.60 | 17.50% |
| Citalopram | Oral | Tablet 20 mg (as hydrobromide) | 3.56 | 43.75% | 2.00 | 2.43 | 17.70% |
| Citalopram | Oral | Tablet 40 mg (as hydrobromide) | 6.03 | 43.75% | 3.39 | 4.11 | 17.52% |
| Clarithromycin | Oral | Powder for oral liquid 250 mg per 5 mL, 50 mL | 14.98 | 15.59% | 12.64 | 14.98 | 15.62% |
| Clarithromycin | Oral | Tablet 250 mg | 3.32 | 15.59% | 2.80 | 3.32 | 15.66% |
| Clarithromycin | Oral | Tablet 500 mg | 47.30 | 15.59% | 39.93 | 47.30 | 15.58% |
| Clopidogrel | Oral | Tablet 75 mg | 20.47 | 50.34% | 10.17 | 11.52 | 11.72% |
| Clopidogrel | Oral | Tablet 75 mg (as besilate) | 20.47 | 50.34% | 10.17 | 11.52 | 11.72% |
| Clopidogrel | Oral | Tablet 75 mg (as hydrogen sulfate) | 20.47 | 50.34% | 10.17 | 11.52 | 11.72% |
| Clozapine | Oral | Tablet 100 mg | 167.20 | 27.52% | 121.19 | 140.83 | 13.95% |
| Clozapine | Oral | Tablet 200 mg | 334.41 | 27.52% | 242.38 | 281.67 | 13.95% |
| Clozapine | Oral | Tablet 25 mg | 44.59 | 27.52% | 32.32 | 37.56 | 13.95% |
| Clozapine | Oral | Tablet 50 mg | 89.18 | 27.52% | 64.64 | 75.12 | 13.95% |
| Desferrioxamine | Injection | Powder for injection containing desferrioxamine mesylate 2 g | 32.40 | 11.30% | 28.74 | 32.40 | 11.30% |
| Desferrioxamine | Injection | Powder for injection containing desferrioxamine mesylate 500 mg | 81.02 | 11.30% | 71.86 | 81.02 | 11.31% |
| Dexamethasone | Injection | Injection containing dexamethasone sodium phosphate equivalent to 4 mg dexamethasone phosphate in 1 mL | 7.26 | 24.34% | 5.49 | 6.77 | 18.91% |
| Dexamethasone | Injection | Injection containing dexamethasone sodium phosphate equivalent to 8 mg dexamethasone phosphate in 2 mL | 13.19 | 24.34% | 9.98 | 12.30 | 18.86% |
| Donepezil | Oral | Tablet containing donepezil hydrochloride 10 mg | 63.50 | 58.13% | 26.59 | 63.50 | 58.13% |
| Donepezil | Oral | Tablet containing donepezil hydrochloride 5 mg | 63.50 | 58.13% | 26.59 | 63.50 | 58.13% |
| Doxorubicin | Injection/intravesical | Solution for I.V. injection or intravesical administration containing doxorubicin hydrochloride 10 mg in 5 mL single dose vial | 4.61 | 39.07% | 2.81 | 3.72 | 24.46% |
| Doxorubicin | Injection/intravesical | Solution for I.V. injection or intravesical administration containing doxorubicin hydrochloride 100 mg in 50 mL single dose vial | 38.97 | 39.07% | 23.74 | 31.41 | 24.42% |
| Doxorubicin | Injection/intravesical | Solution for I.V. injection or intravesical administration containing doxorubicin hydrochloride 200 mg in 100 mL single dose vial | 77.94 | 39.07% | 47.49 | 62.82 | 24.40% |
| Doxorubicin | Injection/intravesical | Solution for I.V. injection or intravesical administration containing doxorubicin hydrochloride 50 mg in 25 mL single dose vial | 19.49 | 39.07% | 11.88 | 15.71 | 24.38% |
| Duloxetine | Oral | Capsule 30 mg (as hydrochloride) | 21.60 | 35.83% | 13.86 | 21.60 | 35.83% |
| Duloxetine | Oral | Capsule 60 mg (as hydrochloride) | 30.86 | 35.83% | 19.80 | 30.86 | 35.84% |
| Etoposide | Injection | Powder for I.V. infusion 1 g (as phosphate) | 174.62 | 13.39% | 151.24 | 174.62 | 13.39% |
| Etoposide | Injection | Powder for I.V. infusion 100 mg (as phosphate) | 17.47 | 13.39% | 15.13 | 17.47 | 13.39% |
| Etoposide | Injection | Solution for I.V. infusion 100 mg in 5 mL vial | 87.33 | 13.39% | 75.64 | 87.33 | 13.39% |
| Famciclovir | Oral | Tablet 125 mg | 106.07 | 48.06% | 55.09 | 65.13 | 15.42% |
| Famciclovir | Oral | Tablet 250 mg | 106.08 | 48.06% | 55.10 | 65.13 | 15.40% |
| Famciclovir | Oral | Tablet 500 mg | 159.11 | 48.06% | 82.64 | 97.69 | 15.41% |
| Famotidine | Oral | Tablet 20 mg | 4.83 | 15.72% | 4.07 | 4.83 | 15.73% |
| Famotidine | Oral | Tablet 40 mg | 4.83 | 15.72% | 4.07 | 4.83 | 15.73% |
| Filgrastim | Injection | Injection 120 micrograms in 0.2 mL single use pre-filled syringe (Nivestim) | 503.11 | 15.20% | 426.64 | 503.11 | 15.20% |
| Filgrastim | Injection | Injection 300 micrograms in 0.5 mL single use pre-filled syringe (Neupogen) | 1,257.77 | 15.20% | 1,066.59 | 1,257.77 | 15.20% |
| Filgrastim | Injection | Injection 300 micrograms in 0.5 mL single use pre-filled syringe (Nivestim) | 1,257.77 | 15.20% | 1,066.59 | 1,257.77 | 15.20% |
| Filgrastim | Injection | Injection 300 micrograms in 0.5 mL single use pre-filled syringe (TevaGrastim) | 1,257.77 | 15.20% | 1,066.59 | 1,257.77 | 15.20% |
| Filgrastim | Injection | Injection 300 micrograms in 0.5 mL single use pre-filled syringe (Zarzio) | 628.88 | 15.20% | 533.29 | 628.88 | 15.20% |
| Filgrastim | Injection | Injection 300 micrograms in 1 mL | 1,257.77 | 15.20% | 1,066.59 | 1,257.77 | 15.20% |
| Filgrastim | Injection | Injection 480 micrograms in 0.5 mL single use pre-filled syringe (Neupogen) | 2,016.29 | 15.20% | 1,709.81 | 2,016.29 | 15.20% |
| Filgrastim | Injection | Injection 480 micrograms in 0.5 mL single use pre-filled syringe (Nivestim) | 2,016.29 | 15.20% | 1,709.81 | 2,016.29 | 15.20% |
| Filgrastim | Injection | Injection 480 micrograms in 0.5 mL single use pre-filled syringe (Zarzio) | 1,008.14 | 15.20% | 854.90 | 1,008.14 | 15.20% |
| Filgrastim | Injection | Injection 480 micrograms in 0.8 mL single use pre-filled syringe (TevaGrastim) | 2,016.29 | 15.20% | 1,709.81 | 2,016.29 | 15.20% |
| Filgrastim | Injection | Injection 480 micrograms in 1.6 mL | 2,016.29 | 15.20% | 1,709.81 | 2,016.29 | 15.20% |
| Flucloxacillin | Injection | Powder for injection 1 g (as sodium) | 6.25 | 33.35% | 4.17 | 6.25 | 33.28% |
| Flucloxacillin | Injection | Powder for injection 500 mg (as sodium) | 3.99 | 33.35% | 2.66 | 3.99 | 33.33% |
| Fluconazole | Injection | Solution for I.V. infusion 100 mg in 50 mL | 10.26 | 82.26% | 1.82 | 4.81 | 62.16% |
| Fluconazole | Injection | Solution for I.V. infusion 200 mg in 100 mL | 19.32 | 82.26% | 3.43 | 9.06 | 62.14% |
| Fluconazole | Injection | Solution for I.V. infusion 400 mg in 200 mL | 30.40 | 82.26% | 5.39 | 14.25 | 62.18% |
| Fluconazole | Oral | Capsule 100 mg | 43.28 | 29.59% | 30.47 | 35.47 | 14.10% |
| Fluconazole | Oral | Capsule 200 mg | 84.30 | 29.59% | 59.36 | 69.09 | 14.08% |
| Fluconazole | Oral | Capsule 50 mg | 22.22 | 29.59% | 15.65 | 18.21 | 14.06% |
| Fludarabine | Injection | Powder for I.V. injection containing fludarabine phosphate 50 mg | 103.11 | 54.94% | 46.46 | 53.87 | 13.76% |
| Fludarabine | Injection | Solution for I.V. injection 50 mg fludarabine phosphate in 2 mL | 515.60 | 54.94% | 232.33 | 269.40 | 13.76% |
| Fluorouracil | Injection | Injection 1000 mg in 20 mL | 5.45 | 11.38% | 4.83 | 5.45 | 11.38% |
| Fluorouracil | Injection | Injection 2500 mg in 50 mL | 13.62 | 11.38% | 12.07 | 13.62 | 11.38% |
| Fluorouracil | Injection | Injection 500 mg in 10 mL | 16.02 | 11.38% | 14.20 | 16.02 | 11.36% |
| Fluorouracil | Injection | Injection 5000 mg in 100 mL | 27.22 | 11.38% | 24.12 | 27.22 | 11.39% |
| Fluoxetine | Oral | Capsule 20 mg (as hydrochloride) | 7.34 | 28.95% | 5.22 | 5.81 | 10.15% |
| Fluoxetine | Oral | Tablet, dispersible, 20 mg (as hydrochloride) | 7.34 | 28.95% | 5.22 | 5.81 | 10.15% |
| Folinic acid | Injection | Injection containing calcium folinate equivalent to 100 mg folinic acid in 10 mL | 8.38 | 43.14% | 4.76 | 5.49 | 13.30% |
| Folinic acid | Injection | Injection containing calcium folinate equivalent to 1000 mg folinic acid in 100 mL | 83.72 | 43.14% | 47.60 | 54.85 | 13.22% |
| Folinic acid | Injection | Injection containing calcium folinate equivalent to 300 mg folinic acid in 30 mL | 24.49 | 43.14% | 13.93 | 16.05 | 13.21% |
| Folinic acid | Injection | Injection containing calcium folinate equivalent to 50 mg folinic acid in 5 mL | 9.07 | 43.14% | 5.16 | 5.94 | 13.13% |
| Frusemide | Injection | Injection 20 mg in 2 mL | 2.59 | 41.22% | 1.52 | 2.33 | 34.76% |
| Galantamine | Oral | Capsule (prolonged release) 16 mg (as hydrobromide) | 49.06 | 24.27% | 37.15 | 42.41 | 12.40% |
| Galantamine | Oral | Capsule (prolonged release) 24 mg (as hydrobromide) | 58.46 | 24.27% | 44.27 | 50.54 | 12.41% |
| Galantamine | Oral | Capsule (prolonged release) 8 mg (as hydrobromide) | 40.10 | 24.27% | 30.37 | 34.67 | 12.40% |
| Gemcitabine | Injection | Powder for I.V. infusion 1 g (as hydrochloride) | 37.35 | 55.32% | 16.69 | 24.27 | 31.23% |
| Gemcitabine | Injection | Powder for I.V. infusion 2 g (as hydrochloride) | 74.87 | 55.32% | 33.45 | 48.66 | 31.26% |
| Gemcitabine | Injection | Powder for I.V. infusion 200 mg (as hydrochloride) | 7.56 | 55.32% | 3.38 | 4.91 | 31.16% |
| Gemcitabine | Injection | Solution concentrate for I.V. infusion 1 g (as hydrochloride) in 25 mL | 37.35 | 55.32% | 16.69 | 24.27 | 31.23% |
| Gemcitabine | Injection | Solution concentrate for I.V. infusion 1000 mg (as hydrochloride) in 100 mL | 37.35 | 55.32% | 16.69 | 24.27 | 31.23% |
| Gemcitabine | Injection | Solution concentrate for I.V. infusion 2 g (as hydrochloride) in 50 mL | 74.87 | 55.32% | 33.45 | 48.66 | 31.26% |
| Gemcitabine | Injection | Solution concentrate for I.V. infusion 200 mg (as hydrochloride) in 20 mL | 7.56 | 55.32% | 3.38 | 4.91 | 31.16% |
| Gemcitabine | Injection | Solution concentrate for I.V. infusion 200 mg (as hydrochloride) in 5 mL | 7.56 | 55.32% | 3.38 | 4.91 | 31.16% |
| Gemcitabine | Injection | Solution concentrate for I.V. infusion 500 mg (as hydrochloride) in 50 mL | 18.68 | 55.32% | 8.35 | 12.14 | 31.22% |
| Gemcitabine | Injection | Solution for injection 1 g (as hydrochloride) in 26.3 mL | 37.35 | 55.32% | 16.69 | 24.27 | 31.23% |
| Gemcitabine | Injection | Solution for injection 2 g (as hydrochloride) in 52.6 mL | 74.87 | 55.32% | 33.45 | 48.66 | 31.26% |
| Gemcitabine | Injection | Solution for injection 200 mg (as hydrochloride) in 5.3 mL | 7.56 | 55.32% | 3.38 | 4.91 | 31.16% |
| Glimepiride | Oral | Tablet 1 mg | 1.32 | 13.19% | 1.15 | 1.32 | 12.88% |
| Glimepiride | Oral | Tablet 2 mg | 2.53 | 13.19% | 2.20 | 2.53 | 13.04% |
| Glimepiride | Oral | Tablet 3 mg | 3.23 | 13.19% | 2.80 | 3.23 | 13.31% |
| Glimepiride | Oral | Tablet 4 mg | 3.96 | 13.19% | 3.44 | 3.96 | 13.13% |
| Glyceryl trinitrate | Transdermal | Transdermal patch 120 mg | 22.15 | 10.72% | 19.78 | 22.15 | 10.70% |
| Glyceryl trinitrate | Transdermal | Transdermal patch 18 mg | 16.90 | 10.72% | 15.09 | 16.90 | 10.71% |
| Glyceryl trinitrate | Transdermal | Transdermal patch 25 mg | 16.90 | 10.72% | 15.09 | 16.90 | 10.71% |
| Glyceryl trinitrate | Transdermal | Transdermal patch 36 mg | 22.15 | 10.72% | 19.78 | 22.15 | 10.70% |
| Glyceryl trinitrate | Transdermal | Transdermal patch 40 mg | 16.90 | 10.72% | 15.09 | 16.90 | 10.71% |
| Glyceryl trinitrate | Transdermal | Transdermal patch 50 mg | 22.15 | 10.72% | 19.78 | 22.15 | 10.70% |
| Glyceryl trinitrate | Transdermal | Transdermal patch 54 mg | 22.15 | 10.72% | 19.78 | 22.15 | 10.70% |
| Glyceryl trinitrate | Transdermal | Transdermal patch 80 mg | 22.15 | 10.72% | 19.78 | 22.15 | 10.70% |
| Granisetron | Injection | Concentrated injection 3 mg (as hydrochloride) in 3 mL | 10.09 | 60.58% | 3.98 | 5.56 | 28.42% |
| Idarubicin | Injection | Solution for I.V. injection containing idarubicin hydrochloride 10 mg in 10 mL | 272.32 | 45.45% | 148.55 | 272.32 | 45.45% |
| Idarubicin | Injection | Solution for I.V. injection containing idarubicin hydrochloride 5 mg in 5 mL | 140.84 | 45.45% | 76.83 | 140.84 | 45.45% |
| Irbesartan | Oral | Tablet 150 mg | 7.80 | 39.06% | 4.75 | 7.80 | 39.10% |
| Irbesartan | Oral | Tablet 300 mg | 15.58 | 39.06% | 9.49 | 15.58 | 39.09% |
| Irbesartan | Oral | Tablet 75 mg | 5.46 | 39.06% | 3.33 | 5.46 | 39.01% |
| Irbesartan with hydrochlorothiazide | Oral | Tablet 150 mg-12.5 mg | 9.31 | 38.06% | 5.77 | 9.31 | 38.02% |
| Irbesartan with hydrochlorothiazide | Oral | Tablet 300 mg-12.5 mg | 17.09 | 38.06% | 10.59 | 17.09 | 38.03% |
| Irbesartan with hydrochlorothiazide | Oral | Tablet 300 mg-25 mg | 18.61 | 38.06% | 11.53 | 18.61 | 38.04% |
| Irinotecan | Injection | I.V. injection containing irinotecan hydrochloride trihydrate 100 mg in 5 mL | 27.60 | 50.85% | 13.57 | 22.00 | 38.32% |
| Irinotecan | Injection | I.V. injection containing irinotecan hydrochloride trihydrate 300 mg in 15 mL | 82.73 | 50.85% | 40.66 | 65.95 | 38.35% |
| Irinotecan | Injection | I.V. injection containing irinotecan hydrochloride trihydrate 40 mg in 2 mL | 11.04 | 50.85% | 5.43 | 8.80 | 38.30% |
| Irinotecan | Injection | I.V. injection containing irinotecan hydrochloride trihydrate 500 mg in 25 mL | 141.44 | 50.85% | 69.52 | 112.76 | 38.35% |
| Iron polymaltose complex | Injection | Injection 100 mg (iron) in 2 mL | 26.00 | 21.88% | 20.31 | 26.00 | 21.88% |
| Isotretinoin | Oral | Capsule 10 mg | 43.32 | 21.47% | 34.02 | 38.44 | 11.50% |
| Isotretinoin | Oral | Capsule 20 mg | 67.52 | 21.47% | 53.02 | 59.91 | 11.50% |
| Isotretinoin | Oral | Capsule 40 mg | 60.94 | 21.47% | 47.86 | 54.07 | 11.49% |
| Lamivudine | Oral | Oral solution 10 mg per mL, 240 mL | 86.48 | 35.27% | 55.98 | 72.86 | 23.17% |
| Lamivudine | Oral | Oral solution 5 mg per mL, 240 mL | 69.91 | 35.27% | 45.25 | 58.90 | 23.17% |
| Lamivudine | Oral | Tablet 100 mg | 125.47 | 35.27% | 81.22 | 105.71 | 23.17% |
| Lamivudine | Oral | Tablet 150 mg | 236.89 | 35.27% | 153.34 | 199.58 | 23.17% |
| Lamivudine | Oral | Tablet 300 mg | 236.89 | 35.27% | 153.34 | 199.58 | 23.17% |
| Lamivudine with zidovudine | Oral | Tablet 150 mg-300 mg | 484.17 | 18.68% | 393.73 | 484.17 | 18.68% |
| Letrozole | Oral | Tablet 2.5 mg | 111.71 | 35.85% | 71.66 | 94.16 | 23.90% |
| Meloxicam | Oral | Capsule 15 mg | 9.95 | 35.81% | 6.39 | 7.22 | 11.50% |
| Meloxicam | Oral | Capsule 7.5 mg | 6.82 | 35.81% | 4.38 | 4.95 | 11.52% |
| Meloxicam | Oral | Tablet 15 mg | 9.95 | 35.81% | 6.39 | 7.22 | 11.50% |
| Meloxicam | Oral | Tablet 7.5 mg | 6.82 | 35.81% | 4.38 | 4.95 | 11.52% |
| Metoprolol | Oral | Tablet containing metoprolol tartrate 100 mg | 3.13 | 10.12% | 2.81 | 3.13 | 10.22% |
| Metoprolol | Oral | Tablet containing metoprolol tartrate 50 mg | 2.45 | 10.12% | 2.20 | 2.45 | 10.20% |
| Metronidazole | Injection | I.V. infusion 500 mg in 100 mL | 2.18 | 53.60% | 1.01 | 1.20 | 15.83% |
| Mirtazapine | Oral | Tablet 15 mg | 5.96 | 37.64% | 3.72 | 4.32 | 13.89% |
| Mirtazapine | Oral | Tablet 15 mg (orally disintegrating) | 10.29 | 37.64% | 6.42 | 7.46 | 13.94% |
| Mirtazapine | Oral | Tablet 30 mg | 8.95 | 37.64% | 5.58 | 6.49 | 14.02% |
| Mirtazapine | Oral | Tablet 30 mg (orally disintegrating) | 13.72 | 37.64% | 8.56 | 9.94 | 13.88% |
| Mirtazapine | Oral | Tablet 45 mg | 14.93 | 37.64% | 9.31 | 10.82 | 13.96% |
| Mirtazapine | Oral | Tablet 45 mg (orally disintegrating) | 20.68 | 37.64% | 12.90 | 14.98 | 13.89% |
| Montelukast | Oral | Tablet, chewable, 4 mg (as sodium) | 28.91 | 29.33% | 20.43 | 28.91 | 29.33% |
| Montelukast | Oral | Tablet, chewable, 5 mg (as sodium) | 27.18 | 29.33% | 19.21 | 27.18 | 29.32% |
| Mycophenolic acid | Oral | Capsule containing mycophenolate mofetil 250 mg | 70.07 | 35.30% | 45.34 | 58.48 | 22.47% |
| Mycophenolic acid | Oral | Tablet (enteric coated) containing mycophenolate sodium equivalent to 180 mg mycophenolic acid | 168.18 | 35.30% | 108.81 | 140.36 | 22.48% |
| Mycophenolic acid | Oral | Tablet (enteric coated) containing mycophenolate sodium equivalent to 360 mg mycophenolic acid | 336.34 | 35.30% | 217.61 | 280.71 | 22.48% |
| Mycophenolic acid | Oral | Tablet containing mycophenolate mofetil 500 mg | 140.14 | 35.30% | 90.67 | 116.96 | 22.48% |
| Nitrazepam | Oral | Tablet 5 mg | 1.38 | 7.30% | 1.28 | 1.50 | 14.67% |
| Norfloxacin | Oral | Tablet 400 mg | 4.94 | 24.27% | 3.74 | 4.22 | 11.37% |
| Olanzapine | Oral | Tablet 10 mg | 109.41 | 56.42% | 47.68 | 73.69 | 35.30% |
| Olanzapine | Oral | Tablet 10 mg (as benzoate) | 109.41 | 56.42% | 47.68 | 73.69 | 35.30% |
| Olanzapine | Oral | Tablet 10 mg (orally disintegrating) | 109.41 | 56.42% | 47.68 | 73.69 | 35.30% |
| Olanzapine | Oral | Tablet 15 mg (orally disintegrating) | 164.12 | 56.42% | 71.52 | 110.53 | 35.29% |
| Olanzapine | Oral | Tablet 2.5 mg | 27.35 | 56.42% | 11.92 | 18.42 | 35.29% |
| Olanzapine | Oral | Tablet 2.5 mg (as benzoate) | 27.35 | 56.42% | 11.92 | 18.42 | 35.29% |
| Olanzapine | Oral | Tablet 20 mg (orally disintegrating) | 218.81 | 56.42% | 95.36 | 147.37 | 35.29% |
| Olanzapine | Oral | Tablet 5 mg | 54.15 | 56.42% | 23.60 | 36.47 | 35.29% |
| Olanzapine | Oral | Tablet 5 mg (as benzoate) | 54.15 | 56.42% | 23.60 | 36.47 | 35.29% |
| Olanzapine | Oral | Tablet 5 mg (orally disintegrating) | 54.15 | 56.42% | 23.60 | 36.47 | 35.29% |
| Olanzapine | Oral | Tablet 7.5 mg | 82.07 | 56.42% | 35.77 | 55.27 | 35.28% |
| Olanzapine | Oral | Tablet 7.5 mg (as benzoate) | 82.07 | 56.42% | 35.77 | 55.27 | 35.28% |
| Olanzapine | Oral | Wafer 10 mg | 109.41 | 56.42% | 47.68 | 73.69 | 35.30% |
| Olanzapine | Oral | Wafer 15 mg | 164.12 | 56.42% | 71.52 | 110.53 | 35.29% |
| Olanzapine | Oral | Wafer 20 mg | 218.81 | 56.42% | 95.36 | 147.37 | 35.29% |
| Olanzapine | Oral | Wafer 5 mg | 54.15 | 56.42% | 23.60 | 36.47 | 35.29% |
| Ondansetron | Injection | I.V. injection 4 mg (as hydrochloride dihydrate) in 2 mL | 1.93 | 53.16% | 0.90 | 1.12 | 19.64% |
| Ondansetron | Injection | I.V. injection 8 mg (as hydrochloride dihydrate) in 4 mL | 3.06 | 53.16% | 1.43 | 1.78 | 19.66% |
| Ondansetron | Oral | Tablet (orally disintegrating) 4 mg | 16.17 | 49.13% | 8.23 | 9.32 | 11.70% |
| Ondansetron | Oral | Tablet (orally disintegrating) 8 mg | 25.33 | 49.13% | 12.89 | 14.59 | 11.65% |
| Ondansetron | Oral | Tablet 4 mg (as hydrochloride dihydrate) | 16.17 | 49.13% | 8.23 | 9.32 | 11.70% |
| Ondansetron | Oral | Tablet 8 mg (as hydrochloride dihydrate) | 25.33 | 49.13% | 12.89 | 14.59 | 11.65% |
| Ondansetron | Oral | Wafer 4 mg | 16.17 | 49.13% | 8.23 | 9.32 | 11.70% |
| Ondansetron | Oral | Wafer 8 mg | 25.33 | 49.13% | 12.89 | 14.59 | 11.65% |
| Oxaliplatin | Injection | Powder for I.V. infusion 100 mg | 79.14 | 76.33% | 18.73 | 31.74 | 40.99% |
| Oxaliplatin | Injection | Powder for I.V. infusion 50 mg | 40.97 | 76.33% | 9.70 | 16.43 | 40.96% |
| Oxaliplatin | Injection | Solution concentrate for I.V. infusion 100 mg in 20 mL | 79.14 | 76.33% | 18.73 | 31.74 | 40.99% |
| Oxaliplatin | Injection | Solution concentrate for I.V. infusion 200 mg in 40 mL | 157.74 | 76.33% | 37.34 | 63.27 | 40.98% |
| Oxaliplatin | Injection | Solution concentrate for I.V. infusion 50 mg in 10 mL | 40.97 | 76.33% | 9.70 | 16.43 | 40.96% |
| Paclitaxel | Injection | Solution concentrate for I.V. infusion 100 mg in 16.7 mL | 34.00 | 53.80% | 15.71 | 21.81 | 27.97% |
| Paclitaxel | Injection | Solution concentrate for I.V. infusion 150 mg in 25 mL | 49.57 | 53.80% | 22.90 | 31.80 | 27.99% |
| Paclitaxel | Injection | Solution concentrate for I.V. infusion 30 mg in 5 mL | 10.25 | 53.80% | 4.74 | 6.58 | 27.96% |
| Paclitaxel | Injection | Solution concentrate for I.V. infusion 300 mg in 50 mL | 103.29 | 53.80% | 47.72 | 66.27 | 27.99% |
| Pamidronic acid | Injection | Concentrated injection containing disodium pamidronate 15 mg in 5 mL | 26.24 | 23.16% | 20.16 | 22.83 | 11.70% |
| Pamidronic acid | Injection | Concentrated injection containing disodium pamidronate 30 mg in 10 mL | 52.48 | 23.16% | 40.33 | 45.67 | 11.69% |
| Pamidronic acid | Injection | Concentrated injection containing disodium pamidronate 60 mg in 10 mL | 104.95 | 23.16% | 80.64 | 91.33 | 11.70% |
| Pamidronic acid | Injection | Concentrated injection containing disodium pamidronate 90 mg in 10 mL | 157.43 | 23.16% | 120.97 | 137.00 | 11.70% |
| Pamidronic acid | Injection | Injection set containing 1 vial powder for I.V. infusion containing disodium pamidronate 90 mg and 1 ampoule solvent 10 mL | 157.43 | 23.16% | 120.97 | 137.00 | 11.70% |
| Pamidronic acid | Injection | Injection set containing 2 vials powder for I.V. infusion containing disodium pamidronate 30 mg and 2 ampoules solvent 10 mL | 104.96 | 23.16% | 80.65 | 91.34 | 11.70% |
| Paroxetine | Oral | Tablet 20 mg (as hydrochloride) | 6.31 | 27.31% | 4.59 | 5.29 | 13.23% |
| Paroxetine | Oral | Tablet 20 mg (as mesilate) | 6.31 | 27.31% | 4.59 | 5.29 | 13.23% |
| Pioglitazone | Oral | Tablet 15 mg (as hydrochloride) | 27.74 | 35.79% | 17.81 | 20.52 | 13.21% |
| Pioglitazone | Oral | Tablet 30 mg (as hydrochloride) | 42.67 | 35.79% | 27.40 | 31.56 | 13.18% |
| Pioglitazone | Oral | Tablet 45 mg (as hydrochloride) | 55.48 | 35.79% | 35.62 | 41.04 | 13.21% |
| Pravastatin | Oral | Tablet containing pravastatin sodium 10 mg | 3.84 | 37.46% | 2.40 | 2.81 | 14.59% |
| Pravastatin | Oral | Tablet containing pravastatin sodium 20 mg | 6.10 | 37.46% | 3.81 | 4.46 | 14.57% |
| Pravastatin | Oral | Tablet containing pravastatin sodium 40 mg | 9.50 | 37.46% | 5.94 | 6.94 | 14.41% |
| Pravastatin | Oral | Tablet containing pravastatin sodium 80 mg | 14.60 | 37.46% | 9.13 | 10.67 | 14.43% |
| Prazosin | Oral | Tablet 1 mg (as hydrochloride) | 4.00 | 14.14% | 3.43 | 4.00 | 14.25% |
| Prazosin | Oral | Tablet 2 mg (as hydrochloride) | 6.37 | 14.14% | 5.47 | 6.37 | 14.13% |
| Prazosin | Oral | Tablet 5 mg (as hydrochloride) | 11.44 | 14.14% | 9.82 | 11.44 | 14.16% |
| Prochlorperazine | Oral | Tablet containing prochlorperazine maleate 5 mg | 1.83 | 28.44% | 1.31 | 1.47 | 10.88% |
| Quetiapine | Oral | Tablet (modified release) 150 mg (as fumarate) | 83.85 | 38.67% | 51.43 | 67.88 | 24.23% |
| Quetiapine | Oral | Tablet (modified release) 200 mg (as fumarate) | 114.25 | 38.67% | 70.07 | 92.49 | 24.24% |
| Quetiapine | Oral | Tablet (modified release) 300 mg (as fumarate) | 167.63 | 38.67% | 102.81 | 135.71 | 24.24% |
| Quetiapine | Oral | Tablet (modified release) 400 mg (as fumarate) | 228.50 | 38.67% | 140.14 | 184.99 | 24.24% |
| Quetiapine | Oral | Tablet (modified release) 50 mg (as fumarate) | 59.26 | 38.67% | 36.34 | 47.97 | 24.24% |
| Quetiapine | Oral | Tablet 100 mg (as fumarate) | 83.85 | 38.67% | 51.43 | 67.88 | 24.23% |
| Quetiapine | Oral | Tablet 200 mg (as fumarate) | 114.25 | 38.67% | 70.07 | 92.49 | 24.24% |
| Quetiapine | Oral | Tablet 25 mg (as fumarate) | 29.63 | 38.67% | 18.17 | 23.99 | 24.26% |
| Quetiapine | Oral | Tablet 300 mg (as fumarate) | 167.63 | 38.67% | 102.81 | 135.71 | 24.24% |
| Rabeprazole | Oral | Tablet containing rabeprazole sodium 10 mg (enteric coated) | 18.26 | 52.89% | 8.60 | 11.15 | 22.87% |
| Rabeprazole | Oral | Tablet containing rabeprazole sodium 20 mg (enteric coated) | 18.26 | 52.89% | 8.60 | 11.15 | 22.87% |
| Ramipril | Oral | Capsule 1.25 mg | 1.98 | 26.91% | 1.45 | 1.64 | 11.59% |
| Ramipril | Oral | Capsule 10 mg | 6.42 | 26.91% | 4.69 | 5.31 | 11.68% |
| Ramipril | Oral | Capsule 2.5 mg | 2.90 | 26.91% | 2.12 | 2.40 | 11.67% |
| Ramipril | Oral | Capsule 5 mg | 3.64 | 26.91% | 2.66 | 3.01 | 11.63% |
| Ramipril | Oral | Pack containing 7 tablets 2.5 mg, 21 tablets 5 mg and 10 capsules 10 mg | 5.96 | 26.91% | 4.36 | 4.93 | 11.56% |
| Ramipril | Oral | Tablet 1.25 mg | 1.98 | 26.91% | 1.45 | 1.64 | 11.59% |
| Ramipril | Oral | Tablet 10 mg | 6.42 | 26.91% | 4.69 | 5.31 | 11.68% |
| Ramipril | Oral | Tablet 2.5 mg | 2.90 | 26.91% | 2.12 | 2.40 | 11.67% |
| Ramipril | Oral | Tablet 5 mg | 3.64 | 26.91% | 2.66 | 3.01 | 11.63% |
| Risedronic acid | Oral | Tablet (enteric coated) containing risedronate sodium 35 mg | 32.06 | 10.50% | 28.69 | 32.06 | 10.51% |
| Risedronic acid | Oral | Tablet containing risedronate sodium 150 mg | 34.74 | 10.50% | 31.09 | 34.74 | 10.51% |
| Risedronic acid | Oral | Tablet containing risedronate sodium 30 mg | 218.91 | 10.50% | 195.92 | 218.91 | 10.50% |
| Risedronic acid | Oral | Tablet containing risedronate sodium 35 mg | 32.06 | 10.50% | 28.69 | 32.06 | 10.51% |
| Risedronic acid | Oral | Tablet containing risedronate sodium 5 mg | 32.06 | 10.50% | 28.69 | 32.06 | 10.51% |
| Risperidone | Oral | Tablet 0.5 mg | 3.28 | 36.89% | 2.07 | 2.42 | 14.46% |
| Risperidone | Oral | Tablet 0.5 mg (orally disintegrating) | 5.51 | 36.89% | 3.48 | 4.06 | 14.29% |
| Risperidone | Oral | Tablet 1 mg | 18.98 | 36.89% | 11.98 | 14.00 | 14.43% |
| Risperidone | Oral | Tablet 1 mg (orally disintegrating) | 11.02 | 36.89% | 6.95 | 8.13 | 14.51% |
| Risperidone | Oral | Tablet 2 mg | 43.42 | 36.89% | 27.40 | 32.03 | 14.46% |
| Risperidone | Oral | Tablet 2 mg (orally disintegrating) | 22.13 | 36.89% | 13.97 | 16.33 | 14.45% |
| Risperidone | Oral | Tablet 3 mg | 66.95 | 36.89% | 42.25 | 49.39 | 14.46% |
| Risperidone | Oral | Tablet 3 mg (orally disintegrating) | 32.89 | 36.89% | 20.76 | 24.26 | 14.43% |
| Risperidone | Oral | Tablet 4 mg | 90.38 | 36.89% | 57.04 | 66.67 | 14.44% |
| Risperidone | Oral | Tablet 4 mg (orally disintegrating) | 43.82 | 36.89% | 27.65 | 32.33 | 14.48% |
| Rosuvastatin | Oral | Tablet 10 mg (as calcium) | 30.48 | 36.46% | 19.37 | 30.48 | 36.45% |
| Rosuvastatin | Oral | Tablet 20 mg (as calcium) | 43.40 | 36.46% | 27.58 | 43.40 | 36.45% |
| Rosuvastatin | Oral | Tablet 40 mg (as calcium) | 61.90 | 36.46% | 39.33 | 61.90 | 36.46% |
| Rosuvastatin | Oral | Tablet 5 mg (as calcium) | 20.77 | 36.46% | 13.20 | 20.77 | 36.45% |
| Roxithromycin | Oral | Tablet 150 mg | 2.16 | 17.51% | 1.78 | 2.16 | 17.59% |
| Roxithromycin | Oral | Tablet 300 mg | 2.16 | 17.51% | 1.78 | 2.16 | 17.59% |
| Sertraline | Oral | Tablet 100 mg (as hydrochloride) | 4.73 | 41.80% | 2.75 | 3.15 | 12.70% |
| Sertraline | Oral | Tablet 50 mg (as hydrochloride) | 4.73 | 41.80% | 2.75 | 3.15 | 12.70% |
| Simvastatin | Oral | Tablet 10 mg | 3.51 | 49.50% | 1.77 | 2.11 | 16.11% |
| Simvastatin | Oral | Tablet 20 mg | 5.06 | 49.50% | 2.56 | 3.04 | 15.79% |
| Simvastatin | Oral | Tablet 40 mg | 7.28 | 49.50% | 3.68 | 4.37 | 15.79% |
| Simvastatin | Oral | Tablet 5 mg | 2.54 | 49.50% | 1.28 | 1.52 | 15.79% |
| Simvastatin | Oral | Tablet 80 mg | 10.46 | 49.50% | 5.28 | 6.28 | 15.92% |
| Sodium lactate compound | Injection | I.V. infusion containing approximately 131 mmol sodium (as lactate and chloride), 5 mmol potassium (as chloride), 2 mmol calcium (as chloride), 29 mmol bicarbonate (as lactate) and 111 mmol chloride per L, 1 L | 1.47 | 13.72% | 1.27 | 1.47 | 13.61% |
| Sodium lactate compound | Injection | I.V. infusion containing approximately 65 mmol sodium (as lactate and chloride), 2.7 mmol potassium (as chloride), 0.9 mmol calcium (as chloride), 14 mmol bicarbonate (as lactate) and 56 mmol chloride per 500 mL, 500 mL | 1.03 | 13.72% | 0.89 | 1.03 | 13.59% |
| Sumatriptan | Oral | Tablet (fast disintegrating) 50 mg (as succinate) | 6.09 | 36.59% | 3.86 | 4.66 | 17.17% |
| Sumatriptan | Oral | Tablet 50 mg (as succinate) | 6.09 | 36.59% | 3.86 | 4.66 | 17.17% |
| Temazepam | Oral | Tablet 10 mg | 0.84 | 11.22% | 0.75 | 0.84 | 10.71% |
| Temozolomide | Oral | Capsule 100 mg | 518.23 | 26.74% | 379.66 | 455.17 | 16.59% |
| Temozolomide | Oral | Capsule 140 mg | 714.69 | 26.74% | 523.58 | 627.72 | 16.59% |
| Temozolomide | Oral | Capsule 180 mg | 865.42 | 26.74% | 634.01 | 795.30 | 20.28% |
| Temozolomide | Oral | Capsule 20 mg | 120.97 | 26.74% | 88.62 | 106.25 | 16.59% |
| Temozolomide | Oral | Capsule 250 mg | 1,231.27 | 26.74% | 902.03 | 1,081.44 | 16.59% |
| Temozolomide | Oral | Capsule 5 mg | 41.15 | 26.74% | 30.15 | 36.14 | 16.57% |
| Topotecan | Injection | Powder for I.V. infusion 4 mg (as hydrochloride) | 330.40 | 65.21% | 114.95 | 145.77 | 21.14% |
| Tropisetron | Injection | I.V. injection 5 mg (as hydrochloride) in 5 mL | 18.50 | 47.19% | 9.77 | 18.50 | 47.19% |
| Venlafaxine | Oral | Capsule (modified release) 150 mg (as hydrochloride) | 25.96 | 51.74% | 12.53 | 18.61 | 32.67% |
| Venlafaxine | Oral | Capsule (modified release) 37.5 mg (as hydrochloride) | 12.06 | 51.74% | 5.82 | 8.65 | 32.72% |
| Venlafaxine | Oral | Capsule (modified release) 75 mg (as hydrochloride) | 21.29 | 51.74% | 10.27 | 15.26 | 32.70% |
Confirmed prices of brands scheduled for a price disclosure reduction
The Confirmed Prices Report outlines the approved ex-manufacturer prices for brands scheduled to take a price disclosure reduction. The reports for the 2014 October cycle can be downloaded below.




