Exenatide, pre-filled injection pen, 5 microgram per dose, 10 microgram per dose, 60 doses, Byetta, March 2008
Public summary document for Exenatide, pre-filled injection pen, 5 microgram per dose, 10 microgram per dose, 60 doses, Byetta, March 2008
Page last updated: 04 July 2008
Public Summary Documents
Product: Exenatide, pre-filled injection pen, 5 microgram per dose, 10 microgram per
dose, 60 doses, Byetta
Sponsor: Eli Lilly Australia Pty Ltd
Date of PBAC Consideration: March 2008
1. Purpose of Application
To seek an Authority Required listing as adjunctive therapy in patients with type 2 diabetes no longer achieving glycaemic control despite optimal therapy with metformin and/or a sulfonylurea.
2. Background
Exenatide was first considered by the PBAC at its July 2007 meeting. The PBAC rejected the submission on the grounds of high and uncertain cost-effectiveness against the comparators in the absence of any evidence of incremental clinical benefit other than the observational finding of weight loss, which has not been shown to be durable or to translate into morbidity or mortality benefits, and because of unresolved safety concerns.
3. Registration Status
4. Listing Requested and PBAC’s View
Authority required
Combination therapy with metformin and a sulfonylurea
Initiation of therapy, in combination with metformin and a sulfonylurea, in type 2
diabetes mellitus patients who have an HbA1c greater than 7% despite maximally tolerated
doses of metformin and a sulfonylurea.
The date of the HbA1c measurement, which must be no greater than 4 months old at the
time of application, must be provided.
Continuation of therapy, in combination with metformin and a sulfonylurea, in type
2 diabetes mellitus patients where the patient has previously been issued with an
authority prescription for exenatide.
Authority required
Combination therapy with metformin or a sulfonylurea
Initiation of therapy, in combination with either metformin or a sulfonylurea, in
type 2 diabetes mellitus patients who have an HbA1c greater than 7% and in whom a
combination of metformin and a sulfonylurea is contraindicated or not tolerated.
The date of the HbA1c measurement, which must be no greater than 4 months old at the
time of application, must be provided.
Continuation of therapy, in combination with either metformin or a sulfonylurea, in
type 2 diabetes mellitus patients where the patient has previously been issued with
an authority prescription for exenatide.
5. Clinical Place for the Proposed Therapy
Exenatide provides an alternative class of drug for adjunctive therapy in adults with type 2 diabetes who are taking metformin or a sulfonylurea, or a combination of metformin and a sulfonylurea but are not achieving adequate glycaemic control.
6. Comparator
7. Clinical Trials
The re-submission presented two direct randomised trials comparing exenatide with
insulin glargine (GWAA and GWAO) and four supplementary randomised trials (3 placebo
controlled trials: 112, 113 and 115; and 1 trial versus insulin aspart: GWAD). Four
randomised trials of rosiglitazone (3 compared with placebo: Fonesca et al 2000, Rosenstock
et al 2006 and Daily et al 2004; and one compared with insulin glargine: Rosenstock
et al 2006b) were included to enable an indirect comparison.
The table below lists the trials as published at the time of submission.
|
Trial ID/First author |
Publication title |
Publication citation |
|---|---|---|
|
Randomised trials comparing exenatide with placebo |
||
|
112 |
Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. |
Diabetes Care 2005; 28:1092-1100. |
|
113 |
Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. |
Diabetes Care 2004 ; 27 :2628-2653. |
|
115 |
Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. |
Diabetes Care 2005; 28:1083-1091. |
|
Randomised trials comparing exenatide with insulin glargine |
||
|
GWAA |
Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. |
Annals of Internal Medicine 2005; 143:559-569. |
|
Randomised trials comparing exenatide with insulin aspart |
||
|
GWAD |
A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. |
Diabetologia 2007; 50:259-267. |
|
Randomised trials comparing rosiglitazone with placebo |
||
|
Fonseca V |
Effect of metformin and rosiglitazone combination therapy in patients with type 2 diabetes mellitus: a randomized controlled trial. |
JAMA 2000; 283:1695-1702.Erratum JAMA 2000; 284:1384. |
|
Rosenstock J |
Effect of early addition of rosiglitazone to sulfonylurea in older type 2 diabetes mellitus patients (>60 years): the Rosiglitazone Early vs Sulfonylurea Titration (RESULT) study. |
Diabetes, Obesity and Metabolism 2006; 8:49-57. |
|
Dailey GE |
Glycemic control with glyburide/metformin tablets in combination with rosiglitazone in patients with type 2 diabetes: a randomized, double-blind trial. |
American Journal of Medicine 2004; 116:223-229. |
|
Randomised trials comparing rosiglitazone with insulin glargine |
||
|
Rosenstock J |
Triple therapy in type 2 diabetes: insulin glargine or rosiglitazone added to combination therapy of sulfonylurea plus metformin in insulin-naive patients. |
Diabetes Care 2006; 29:554-559. |
8. Results of Trials
Exenatide versus insulin glargine:
The key results from trials GWAO and GWAA showed no statistically significant difference
between exenatide and insulin glargine for the HbA1c outcomes with a mean change from
baseline and proportion of patients achieving HbA1c ≤7%. Exenatide treatment was associated
with a statistically significant reduction in weight; insulin glargine was associated
with a statistically significant increase in weight; and the difference between treatments
was statistically significant in both the GWAO and GWAA trials, favouring exenatide.
There were no statistically significant differences between exenatide and insulin
glargine on any of the quality of life instruments. Quality of life was assessed in
the insulin-comparator controlled studies, using the following scales:
- Impact on diabetes symptoms: Diabetes Symptom Checklist Revised (DSC-R)
- Treatment satisfaction: Diabetes Treatment Satisfaction Questionnaire status version (DTSQs – GWAA only)
- Impact on treatment flexibility: Diabetes Treatment Flexibility Scale (DTFS - GWAA and GWAD)
- Impact on psychological well-being: Psychological and Well Being Index (PGWB – GWAO only)
- Health-related quality of life: vitality subscale of SF-36, EQ-5D (index score only – GWAA, index + VAS score – GWAD)
- Fear of hypoglycaemic: Hypoglycaemic Fear Survey (GWAO only)
- Treatment evaluation assessment (GWAO only).
There were higher rates of treatment-emergent adverse events and discontinuations
due to adverse events associated with exenatide treatment compared with insulin glargine
in the GWAO and GWAA trials. Insulin glargine was associated with higher rates of
hypoglycaemia. Exenatide was associated with higher rates of nausea.
Exenatide versus rosiglitazone:
The re-submission claimed that insufficient data are available in the published rosiglitazone
trials to enable an indirect comparison of exenatide and rosiglitazone for the outcome
mean change from baseline in HbA1c. The results of the indirect comparison for the
proportion of patients achieving an HbA1c ≤7% indicated no difference between exenatide
and rosiglitazone; and the analysis of mean change from baseline in weight, favoured
exenatide.
The figure below shows how the body weight of the completers vary over the course
of three years. The PBAC considered the 3-year follow-up data was informative. However,
it was not comparative, open-label and may be confounded given it was based on completers
only (patients who dropped out were not included). Uncertainty remained over whether
the results observed at 3 years are sustained over 35 years, which is the time horizon
of the model.
Change in body weight of completers from the placebo-controlled trial 112, 113 and
115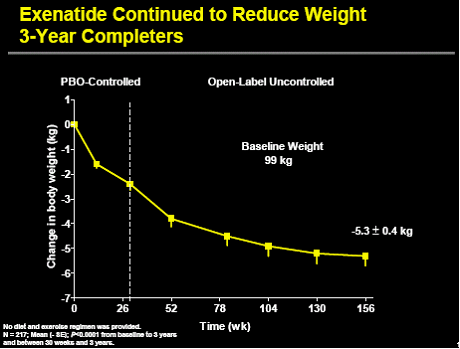
No indirect estimates of the relative safety of exenatide and rosiglitazone were presented.
The PBAC was advised longer-term safety, especially in relation to cardiovascular
complications, remains uncertain with thiazolidinediones and longer term data with
exenatide in this regard would be useful.
9. Clinical Claim
The claim in the re-submission is summarised as follows:
|
Outcome |
Main comparator |
Supportive comparators |
|
|---|---|---|---|
|
Insulin glargine |
Insulin aspart |
Rosiglitazone |
|
|
Clinical effectiveness |
Superior |
Superior |
Superior |
|
- Glycaemic control |
Non-inferior |
Non-inferior |
Non-inferior |
|
- Weight management |
Superior |
Superior |
Superior |
|
Safety |
Equivalent |
Superior |
Equivalent |
|
Overall conclusion |
Superior |
Superior |
Superior |
The PBAC had previously accepted that exenatide is non-inferior to insulin glargine
in terms of its effects on HbA1c. However, the PBAC had concerns about whether the
previous submission adequately demonstrated that exenatide is non-inferior to rosiglitazone
in terms of its effect on HbA1c. The re-submission provided no additional data to
support a claim of non-inferiority to rosiglitazone. There were also residual concerns
regarding the exclusion of a number of potentially relevant rosiglitazone trials.
In terms of weight changes, the exenatide versus insulin glargine trials showed a
statistically significant difference, favouring exenatide. For the comparison with
rosiglitazone, no new data were presented in the re-submission and the PBAC did not
accept the claim of greater weight loss in the previous submission. With respect to
the previous submission, the PBAC had stated that any weight loss benefit has not
been shown to be durable in the longer term.
The PBAC considered that in this re-submission there was no basis for it to change
its view that the weight loss benefit associated with exenatide had not been shown
to be durable in the longer term.
The Committee further noted that no new data had been provided to alter its previous
conclusion that exenatide was associated with a higher incidence of adverse events
versus insulin glargine. A difference in hypoglycaemic events between exenatide and
insulin glargine was also not convincingly demonstrated. Again, no indirect estimates
of the comparative safety of exenatide and rosiglitazone were presented to allow assessment
of the comparative safety of exenatide and rosiglitazone.
10. Economic Analysis
A stepped economic evaluation was presented. It included a modelled economic evaluation
over a time horizon of one year in addition to an updated modelled evaluation over
a time horizon of 35 years.
The inputs of the model were unchanged from the previous submission, in that numerical
differences between exenatide and insulin glargine were modelled in the absence of
statistically significant differences.
The PBAC noted that the modelled evaluation was driven by the valuation of the weight
loss effect of exenatide: changes in body mass index (BMI) which were transformed
into changes in utility values.
Results of the short-term economic evaluation produced an incremental cost per extra
Quality-Adjusted Life Years (QALY) gained in the range of $15,000 to $45,000. Results
of the long-term economic evaluation produced an incremental cost per extra QALY gained
in the range of $15,000 to $45,000 in the base case.
The PBAC considered the results of the modelled evaluation to be uncertain.
11. Estimated PBS Usage and Financial Implications
The likely number of patients per year was estimated to be between 10,000 and 50,000
in Year 5. The estimated net cost per year to the PBS was in the range of $10-30 million
in Year 5.
The re-submission proposed a risk sharing arrangement, in addition to the proposed
Authority Required eligibility criteria.
The submission stated that this proposal was intended to reduce the uncertainty in
the ICERs in relation to the valuation of the weight reduction associated with exenatide.
12. Recommendation and Reasons
The PBAC agreed that the re-submission’s nomination of insulin glargine as the main
comparator was appropriate, noting that in clinical practice it is common to exhaust
all oral alternatives before moving onto an injectable alternative and therefore that
insulin glargine is the treatment most likely to be replaced in clinical practice.
The Committee further agreed that the inclusion of rosiglitazone in a sensitivity
analysis in the modelled economic evaluation was reasonable.
The PBAC noted it has previously accepted that exenatide is non-inferior to insulin
glargine in terms of its effects on HbA1c. However, the re-submission provided no
additional data to address PBAC’s previous concern that the claim of non-inferiority
to rosiglitazone was inadequately demonstrated.
In terms of weight changes, the Committee agreed that the exenatide versus insulin
glargine trials showed a statistically significant difference, favouring exenatide.
However, as before the weight loss benefit associated with exenatide had not been
verified in a properly designed weight loss or quality of life study. Although the
3 year follow-up data on body weight from studies 112, 113 and 115 was informative,
it was open-label and non-comparative and may be confounded as it is based on completers
only (patients who dropped out were not included). The increased nausea observed with
exenatide may contribute to some patients with weight loss shown in the trials. It
was also noted that the pre-PBAC response agrees that “open label data presented beyond
the controlled period should be treated with caution” and notes that there are longer
term prospective trials that will provide comparative data. The PBAC thus considered
there was no basis for it to change its view that the weight loss benefit associated
with exenatide has not been shown to be durable in the longer term.
The Committee further noted that no new data had been provided to alter its previous
conclusion that exenatide was associated with a higher incidence of adverse events
versus insulin glargine. A difference in hypoglycaemic events between exenatide and
insulin glargine was also not convincingly demonstrated. Again, no indirect estimates
of the comparative safety of exenatide and rosiglitazone are presented to allow assessment
of the comparative safety of exenatide and rosiglitazone.
The uncertainties around the clinical benefit of exenatide compared to insulin glargine
(eg whether the weight loss is sustained) result in uncertainty in the outputs of
the economic model.
Additionally, the lactic acidosis sub-model “work around” which is used to handle
changes in body weight, BMI and the associated utilities in the economic model is
still associated with an unacceptable lack of clarity, although the Committee acknowledged
the pre-subcommittee and pre-PBAC response attempts to provide more detail on this
“work-around”. This is illustrated by the implausibly large difference in the estimated
incremental cost effectiveness ratio (ICER) per life year gained compared with the
estimated ICER per quality adjusted life year in a model where weight change is the
primary variable driving the outcome.
The PBAC agreed that overall the data show that weight loss is of value to patients.
However, uncertainty remains as to the size of the benefit; whether the weight loss
is of value in itself or because of impacts on other health outcomes (and what is
captured in the modelling); and whether the weight loss is sustained and how the weight
loss is modelled.
The PBAC did not accept that the uncertainties described above could be managed by
the sponsor’s proposed risk-share arrangement. The Committee considered a more acceptable
approach would be for the sponsor to seek listing on a cost-minimisation basis against
insulin glargine, taking into account potential differences in the adverse event profiles
of the two drugs, and that an insulin glargine dose of 75 IU per day may be able to
be justified in the context of such a request. Price increase requests could then
be made as the data from the new trials become available for review.
Therefore, as previously the Committee rejected the current application on the grounds
of a high and uncertain cost-effectiveness ratio against the comparator, insulin glargine,
in the absence of evidence of clinical benefit other than the observational finding
of weight loss.
13. Context for Decision
The PBAC helps decide whether and, if so, how medicines should be subsidised in Australia. It considers submissions in this context. A PBAC decision not to recommend listing or not to recommend changing a listing does not represent a final PBAC view about the merits of the medicine. A company can resubmit to the PBAC or seek independent review of the PBAC decision.
14. Sponsor’s Comment
The sponsor will make a further submission to the PBAC, keeping in mind the recommendations
for an alternative approach. In regard to the question of the 3 year completers data,
the sponsor acknowledges the limitations of such data but argues that for those patients
who do tolerate and respond to exenatide, this demonstrates sustained glycaemic control
and weight loss. Such patients will have avoided insulin for at least 3 years and
the associated weight gain and, if at high doses of insulin, the associated hypoglycaemic
episodes. The sponsor also notes that although more treatment-emergent adverse events
were reported for exenatide than for insulin glargine in the head to head trials,
the actual discontinuation rate for exenatide remained low.




