Apixaban, tablet, 2.5 mg and 5 mg, Eliquis®
Public Summary Document
Product: Apixaban, tablet, 2.5 mg and 5 mg, Eliquis®
Sponsor: Bristol-Myers Squibb Australia Pty Ltd
Date of PBAC Consideration: November 2012
1. Purpose of Application
The submission sought an Authority Required (Streamlined) listing for the prevention of stroke or systemic embolism in a patient with non-valvular atrial fibrillation (NVAF) who is at moderate-to-high risk of developing stroke or systemic embolism, who meets certain criteria.
This submission was considered under the TGA/PBAC parallel process. TGA documentation was not available during the evaluation, except for the draft product information.
2. Background
The PBAC had not previously considered apixaban for the requested indication.
Apixaban 2.5 mg tablets are currently PBS listed as an Authority Required benefit for prevention of venous thromboembolism in patients undergoing total hip or total knee replacement. Apixaban 5 mg tablets are not currently PBS listed.
3. Registration Status
Apixaban is TGA approved for the following indications:
- Prevention of venous thromboembolic events in adult patients who have undergone elective total hip or total knee replacement surgery; and
- Prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation and at least one additional risk factor for stroke.
4. Listing Requested and PBAC’s View
Authority required (STREAMLINED)
Prevention of stroke or systemic embolism in a patient with non-valvular atrial fibrillation who is at moderate-to-high risk of developing stroke or systemic embolism as evidenced by one or more of the following risk factors:
i. Age 75 years or older;
ii. Hypertension;
iii. Diabetes mellitus;
iv. Heart failure or left ventricular dysfunction (ejection fraction less than 40%);
v. Previous stroke or transient ischaemic attack or systemic embolism.
For PBAC’s view, see Recommendations and Reasons.
5. Clinical Place for the Proposed Therapy
Atrial fibrillation (AF) is a cardiac arrhythmia characterised by uncoordinated atrial activation with consequent deterioration of mechanical function. AF is triggered by atrial premature depolarisations arising in the region of the pulmonary veins and propagates in an irregular and unsynchronised pattern, producing irregularity in the pattern of ventricular activation. The disturbed atrial and ventricular activation creates a hypercoagulable state due to haemostasis in the left atrium which leads to thrombus formation, increasing the risk of stroke and other thrombolic events.
The submission proposed that the place in therapy of apixaban is as an alternative to adjusted-dose warfarin and aspirin as a first line treatment for the prevention of stroke or systemic embolism in moderate-to-high risk patients with NVAF.
6. Comparator
The submission nominated dose-adjusted warfarin and aspirin as the comparators. The submission further nominated dabigatran and rivaroxaban as potential future comparators. This was considered appropriate by the PBAC.
7. Clinical Trials
The submission presented one randomised trial of apixaban (5 mg twice daily (bd) or 2.5 mg bd in some patients) versus dose adjusted warfarin (ARISTOTLE); one randomised trial of apixaban (5 mg bd or 2.5 mg bd in some patients) versus aspirin (81 mg-324 mg/d) (AVERROES), one randomised trial comparing dabigatran (150 mg and 110 mg bd) versus dose adjusted open-label warfarin (RE-LY); and one randomised trial comparing rivaroxaban (20 mg daily, 15 mg daily in some patients) versus dose adjusted warfarin (ROCKET-AF). The results of RE-LY and ROCKET-AF were presented to inform an indirect comparison between apixaban and dabigatran and rivaroxaban respectively, using adjusted dose warfarin as the common reference.
The table below details the published trials presented in the submission.
|
Trial ID/First Author |
Protocol title/ Publication title |
Publication citation |
|---|---|---|
|
Direct randomised trials |
||
|
Apixaban versus dose adjusted warfarin |
||
|
ARISTOTLE |
|
|
|
Granger et al. |
Apixaban versus warfarin in patients with atrial fibrillation |
NEJM(2011); 365:981-92 |
|
Lopes et al. |
Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial: Design and Rationale |
Am Heart J (2010);159:331-339 |
|
Connolly et al. |
Apixaban in patients with atrial fibrillation. |
NEJM (2011); 364(9):806-817.
|
|
Kita et al. |
The ARISTOTLE trial: Apixaban versus warfarin in patients with atrial fibrillation. |
Interventional Cardiology (2012); 3(6):December |
|
Mearns |
Atrial fibrillation: ARISTOTLE reveals superiority of apixaban over warfarin in patients with atrial fibrillation. |
Nature Reviews Cardiology (2012); 8(11):November |
|
Ezekowitz et al. |
Efficacy and safety of apixaban compared to warfarin for prevention of stroke and systemic embolism in 18,201 patients with atrial fibrillation: Primary results of the ARISTOTLE trial. |
Canadian Journal of Cardiology (2012); Conference(var.pagings):September-October |
|
Ogawa et al |
Safety and efficacy of the oral direct factor Xa inhibitor apixaban in Japanese patients with non-valvular atrial fibrillation. -The ARISTOTLE-J study. |
Circulation Journal (2011); 75(8):1852-1859. |
|
Apixaban versus aspirin |
||
|
AVERROES |
|
|
|
Eikelboom et al. |
Rationale and design of AVERROES: Apixaban versus acetylsalicylic acid to prevent stroke in atrial fibrillation patients who have failed or are unsuitable for vitamin K antagonist treatment. |
Am Heart J (2010);159:348-353 |
|
Connolly et al. |
Apixaban in Patients with Atrial Fibrillation |
NEJM (2011); 364:806-81 |
|
De et al. |
[The AVERROES study]. [Italian]. |
Giornale Italiano di Cardiologia (2011); 12(9):551-555 |
|
Diener et al. |
Apixaban versus aspirin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a predefined subgroup analysis from AVERROES, a randomised trial. |
Lancet Neurology (2012); 11(3):225-231 |
|
Lopes et al. |
In people with atrial fibrillation unsuitable for warfarin, apixaban reduces the risk of stroke compared with aspirin, with no difference in major bleeding. |
Evidence-Based Medicine (2012); 16(6):December |
|
Dunn et al. |
Apixaban reduced stroke and systemic embolism compared with aspirin in adults with AF for whom VKA therapy was unsuitable. |
Annals of Internal Medicine (2012); 154(8): April. |
|
Lip et al. |
Impact of treatment with apixaban and aspirin in patients with atrial fibrillation in relation to the CHADS2 and CHA2DS2-vasc scores: The AVARROES study. |
Circulation (2012); Conference (var.pagings):22. |
|
Hohnloser et al. |
Apixaban in patients with atrial fibrillation and their risk for cardiovascular hospitalization: |
Insights from the AVERROES trial. European Heart Journal (2012);[conference abstract] |
|
Eikelboom et al. |
Efficacy and safety of apixaban compared with aspirin in patients with atrial fibrillation who previously used and discontinued warfarin therapy: A secondary analysis of the AVERROES trial. |
European Heart Journal (2012); Conference August 2012 |
|
Hart et al. |
Efficacy and safety of the novel oral factor Xa inhibitor apixaban in atrial fibrillation (AF) patients with chronic kidney disease (CKD): The AVERROES trial. |
European Heart Journal (2012); Conference abstract August 2012 |
|
Diener et al. |
AVERROES: Apixaban versus acetylsalicylic acid (ASA) to prevent strokes. |
Stroke (2012); Conference (var.pagings):01 |
|
Alexander et al. |
European Society of Cardiology: Apixaban or aspirin in decreasing stroke risk (The AVERROES Trial). |
P and T (2012); 35(10):October |
|
Lip et al. |
Impact of Treatment with Apixaban and Aspirin in Patients with Atrial Fibrillation in Relation to the CHADS2 and CHA2DS2-VASc Scores: the AVERROES Study. |
Circulation (2012); 124(21, Suppl S):S. |
|
Ogawa et al. |
Safety and efficacy of the oral direct factor Xa inhibitor apixaban in Japanese patients with non-valvular atrial fibrillation. -The ARISTOTLE-J study. |
Circulation Journal (2011); 75(8):1852-1859. |
|
Indirect comparison: warfarin as common comparator |
||
|
Dabigatran 150 mg and 110 mg versus dose adjusted warfarin |
||
|
RE-LY |
|
|
|
Connolly et al. |
Dabigatran versus warfarin in patients with atrial fibrillation. |
NEJM (2009); 361:1139-51 |
|
Connolly et al. |
Newly identified events in the RE-LY trial. |
NEJM (2010); 363:1875-6 |
|
Ezekowitz et al. |
Rationale and design of RE-LY: Randomised evaluation of long term anticoagulant therapy, warfarin compared with dabigatran. |
Am Heart J (2009); 157:805-810 |
|
Paikin et al. |
Dabigatran for stroke prevention in atrial fibrillation: the RE-LY trial. |
Expert review of cardiovascular therapy (2011); 9 ( 3):279-86
|
|
Hori et al. |
Efficacy and safety of dabigatran vs. warfarin in patients with atrial fibrillation--sub-analysis in Japanese population in RE-LY trial. |
Circulation journal : official journal of the Japanese Circulation Society
|
|
Liesenfeld et al. |
A population pharmacokinetic analysis of the oral thrombin inhibitor dabigatran etexilate in patients with non-valvular atrial fibrillation from the RE-LY trial. |
Journal of thrombosis and haemostasis (2011); 9(11): 2168-75 |
|
Eikelboom et al. |
Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. |
Circulation (2011), 123(21): 2363-72 |
|
Ezekowitz et al. |
Dabigatran and warfarin in vitamin K antagonist-naive and -experienced cohorts with atrial fibrillation. |
Circulation (2010), 122(22): 2246-53 |
|
Diener et al. |
Dabigatran compared with warfarin in patients with atrial fibrillation and previous transient ischaemic attack or stroke: a subgroup analysis of the RE-LY trial. |
Lancet neurology (2010); 9 (12): 1157-63 |
|
Oldgren et al. |
Dabigatran Versus Warfarin In Atrial Fibrillation Patients With Low, Moderate And High Chads2 Score: A RE-LY Subgroup Analysis. |
Journal of the American College of Cardiology (2010); 55 (10A):A1-E2 |
|
Yusuf et al. |
Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE-LY trial. |
Lancet (2010); 376 ( 9745): 975-83 |
|
Khoo et al. |
Insights from the dabigatran versus warfarin in patients with atrial fibrillation (RE-LY) trial. |
Expert opinion on pharmacotherapy (2010); 11 (4): 685-7
|
|
Diener et al. |
Reduced Cerebral Bleeding Rates with Dabigatran Compared to Warfarin in Patients with Atrial Fibrillation: Results of RE-LY |
American Academy of Neurology 62nd Annual Meeting (2010) |
|
Liakishev |
Dabigatran versus Warfarin in patients with atrial fibrillation. Results of the RE-LY study. |
Kardiologiia (2009); 49 (10): 75-6 |
|
Ezekowitz et al |
Rationale and design of RE-LY: randomized evaluation of long-term anticoagulant therapy, warfarin, compared with dabigatran. |
American heart journal (2009); 157 (5): 805-10, 810.e1-2 |
|
Hart et al. |
Intracranial Haemorrhage in Atrial Fibrillation Patients During Anticoagulation With Warfarin or Dabigatran: The RE-LY Trial. |
Stroke (2012); 43(6):1511-7 |
|
Uchiyama |
Expectation to and problems of thrombin inhibitor. [Japanese] |
Rinsho Shinkeigaku - Clinical Neurology (2011); 51(11):1004-6 |
|
Hohnloser et al. |
Myocardial ischemic events in patients with atrial fibrillation treated with dabigatran or warfarin in the RE-LY (Randomized Evaluation of Long-Term Anticoagulation Therapy) trial. |
Circulation (2012); 125(5):669-76 |
|
Oldgren |
Risks for stroke, bleeding, and death in patients with atrial fibrillation receiving dabigatran or warfarin in relation to the CHADS2 score: a subgroup analysis of the RE-LY trial. |
Annals of Internal Medicine (2011) 155(10):660-7 |
|
Nagarakanti et al. |
Dabigatran versus warfarin in patients with atrial fibrillation: an analysis of patients undergoing cardioversion. |
Circulation (2011); 123(2):131-6. |
|
Wallentin et al. |
Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE-LY trial. |
Lancet (2010); 376(9745): 975-83 |
|
Hijazi et al. |
NT-proBNP is Prognostic for Stroke and Death in Atrial Fibrillation - a RELY Sub study |
Circulation (2010) 122(21, Suppl.S): A13472 |
|
Panichpisal et al. |
Dabigatran for stroke prevention in patients with atrial fibrillation and previous stroke or transient ischemic attack: Does dose matter? |
Future Neurology (2011); 6 (2): 155-158 |
|
Ferreira et al. |
Dabigatran compared with warfarin in patients with atrial fibrillation and symptomatic heart failure: A subgroup analysis of the RE-LY trial. |
Circulation. Conference: American Heart Association's Scientific Sessions (2011) Orlando, FL United States. 124 (21 SUPPL. 1)
|
|
Eikelboom et al. |
No evidence of platelet activation in patients with atrial fibrillation who are treated with dabigatran: A sub study of the rely trial. Journal of
|
Thrombosis and Haemostasis. Conference: 23rd Congress of the International Society on Thrombosis and Haemostasis 57th Annual SSC Meeting Kyoto Japan. Conference Start: 2011-07-23 Conference End: 2011-07-28. Conference Publication: 9 (pp 346), 2011. Date of Publication: July 2011 |
|
Siegbahn et al. |
Dabigatran and warfarin affects the coagulation process at different levels during long-term treatment - A RELY sub study. European Heart |
Journal. Conference: European Society of Cardiology, ESC Congress 2011 Paris France. Conference Start: 2011-08-27 Conference End: 2011-08-31. Conference Publication: 32 (pp 467), 2011. Date of Publication: August 2011. |
|
Healey et al |
Effect of age and renal function on the risks of stroke and major bleeding with dabigatran compared to warfarin: An analysis from the RE-LY study. |
Journal of the American College of Cardiology. Conference: American College of Cardiology's 59th Annual Scientific Session and i2 Summit: Innovation in Intervention Atlanta, GA United States. Conference Start: 2010-03-14 Conference End: 2010-03-16. Conference Publication: (var.pagings). 55 (10 SUPPL 1) (pp A4.E37), 2010. Date of Publication: 09 Mar 2010. |
|
Wallentin et al |
Efficacy and safety of dabigatran compared to warfarin at different levels of INR control for stroke prevention in 18,113 patients with atrial fibrillation in the rely trial. |
Circulation. Conference: American Heart Association's Scientific Sessions 2009 Orlando, FL United States. Conference Start: 2009-11-14 Conference End: 2009-11-18. Conference Publication: (var.pagings). 120 (21) (pp 2158), 2010. Date of Publication: 2010. |
|
Diener et al. |
Dabigatran compared to warfarin in patients with atrial fibrillation and prior TIA or stroke: The RE-LY study. |
Journal of Neurology. Conference: 20th Meeting of the European Neurological Society Berlin Germany. Conference Start: 2010-06-19 Conference End: 2010-06-23. Conference Publication: 257 (pp S31), 2010. Date of Publication: June 2010. |
|
Connolly |
RELY: Randomized evaluation of long-term anticoagulant therapy. |
European Journal of Heart Failure (2009); 11(12): 1215 |
|
Eikelboom et al. |
Dabigatran versus warfarin in patients with atrial fibrillation. |
NEJM (2009); 361(12): 1139-1151
|
|
Rivaroxaban versus dose adjusted warfarin |
||
|
ROCKET-AF |
|
|
|
Patel et al. |
Rivaroxaban versus warfarin in nonvalular atrial fibrillation. |
NEJM(2011); 365: 883-891 |
|
ROCKET AF Study Investigators |
Rivaroxaban – once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism in trial in atrial fibrillation: rationale and design of the ROCKET AF Study. |
Am Heart J (2010); 159(3): 340.e1-347.e1. |
|
Fox et al. |
Prevention of stroke and systemic embolism with rivaroxaban compared with warfarin in patients with non-valvular atrial fibrillation and moderate renal impairment. |
European heart journal (2011); 32 (19): 2387-94 |
|
Singer et al. |
Individual and Regional Determinants of Time in Therapeutic Range Among Patients Randomized to Warfarin in the ROCKET AF Trial of Rivaroxaban |
Circulation (2011); 124(21, Suppl. S): A16169 |
|
Mahaffey et al. |
Ischemic Cardiac Outcomes in Patients with AF Treated with Vitamin K Antagonism or Factor Xa Inhibition: Results from the ROCKET AF Trial. |
Circulation (2011); 124(21, Suppl. S):A13482 |
|
Patel et al. |
Stroke Prevention Using the Oral Direct Factor Xa Inhibitor Rivaroxaban Compared With Warfarin in Patients With Nonvalvular Atrial Fibrillation (ROCKET AF). |
Circulation (2010); 122(21):2217
|
|
Patel et al. |
Rivaroxaban-Once daily, oral, direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation: Rationale and Design of the ROCKET AF study. |
American Heart Journal (2010) 159(3):340 |
|
Goodman et al. |
Predictors of major bleeding risk: Insights from the rivaroxaban once-daily oral direct factor XA inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation (Rocket AF). |
Circulation. Conference: American Heart Association's Scientific Sessions 2011 Orlando, FL United States. Conference Start: 2011-11-12 Conference End: 2011-11-16. Conference Publication: (var.pagings). 124 (21 SUPPL. 1), 2011. Date of Publication: 22 Nov 2011. |
|
Hacke et al. |
Rivaroxaban versus warfarin in patients with AF and prior cerebrovascular disease: Results from the rocket-AF trial.
|
Cerebrovascular Diseases. Conference: 20th European Stroke Conference, ESC 2011 Hamburg Germany. Conference Start: 2011-05-24 Conference End: 2011-05-27 Sponsor: Bayer, Boehringer, ev3, Allergan, Pfizer, et al. Conference Publication: 31 (pp 17), 2011. Date of Publication: May 2011. |
|
Hori et al. |
J ROCKET AF: The safety and efficacy of rivaroxaban for prevention of stroke in Japanese patients with non-valvular atrial fibrillation. |
Journal of Thrombosis and Haemostasis. Conference: 23rd Congress of the International Society on Thrombosis and Haemostasis 57th Annual SSC Meeting Kyoto Japan. Conference Start: 2011-07-23 Conference End: 2011-07-28. Conference Publication: 9 (pp 20), 2011. Date of Publication: July 2011. |
|
Hori et al.* |
Rivaroxaban vs. Warfarin in Japanese Patients With Atrial Fibrillation – The J-ROCKET AF Study. |
Circulation Journal, Official Journal of the Japanese Circulation Society. Advanced publication date published (5 June 2012) online only. |
|
Meta-analyses of direct randomised trials |
||
|
Agarwal et al. |
Current Trial-Associated Outcomes With Warfarin in Prevention of Stroke in Patients With Nonvalvular Atrial Fibrillation: A Meta-analysis. |
Arch Intern Med. (2012);172(8):623-631 |
|
Baker et al. |
Do differences exist between oral anticoagulants in patients with nonvalular atrial fibrillation? An adjusted indirect comparison meta-analysis. |
Presented at the 61th Annual Scientific Session of the American College of Cardiology & i2Summit Innovation in Intervention ACC:12 Chicago. Presentation Number: 1235-90 |
|
Miller et al. |
Meta-analysis of efficacy and safety of new oral anticoagulants (Dabigatran, Rivaroxaban, Apixaban) versus Warfarin in patients with atrial fibrillation. |
American Journal of Cardiology (2012); (10.1016/j.amjcard.2012.03.049) |
*indicate citations that were retrieved during the evaluation.
Bolded citation represents the primary publication used throughout the submission
8. Results of Trials
A summary of direct hazard ratio (HR) (or relative risk (RR) when HR not reported) for all patient relevant outcomes of ARISTOTLE (apixaban 5 mg vs. warfarin), RE-LY (dabigatran 110mg vs warfarin), RE-LY (dabigatran 150 mg vs warfarin), ROCKET (rivaroxaban 20 mg vs. warfarin) is shown in the figure below.
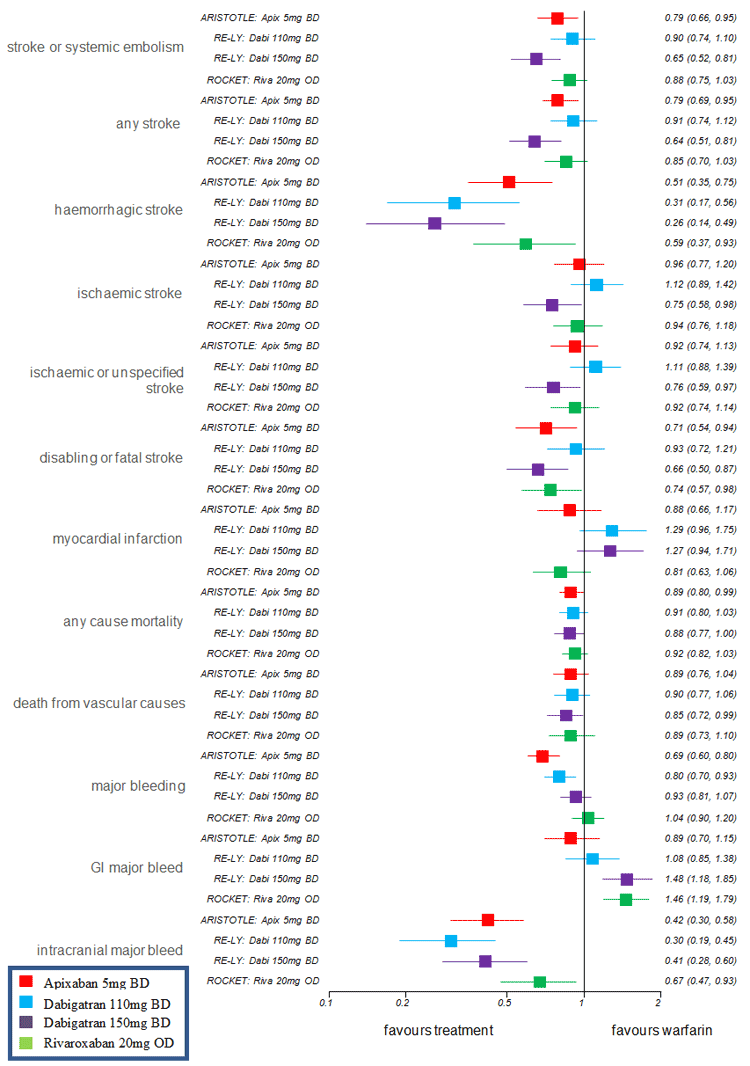
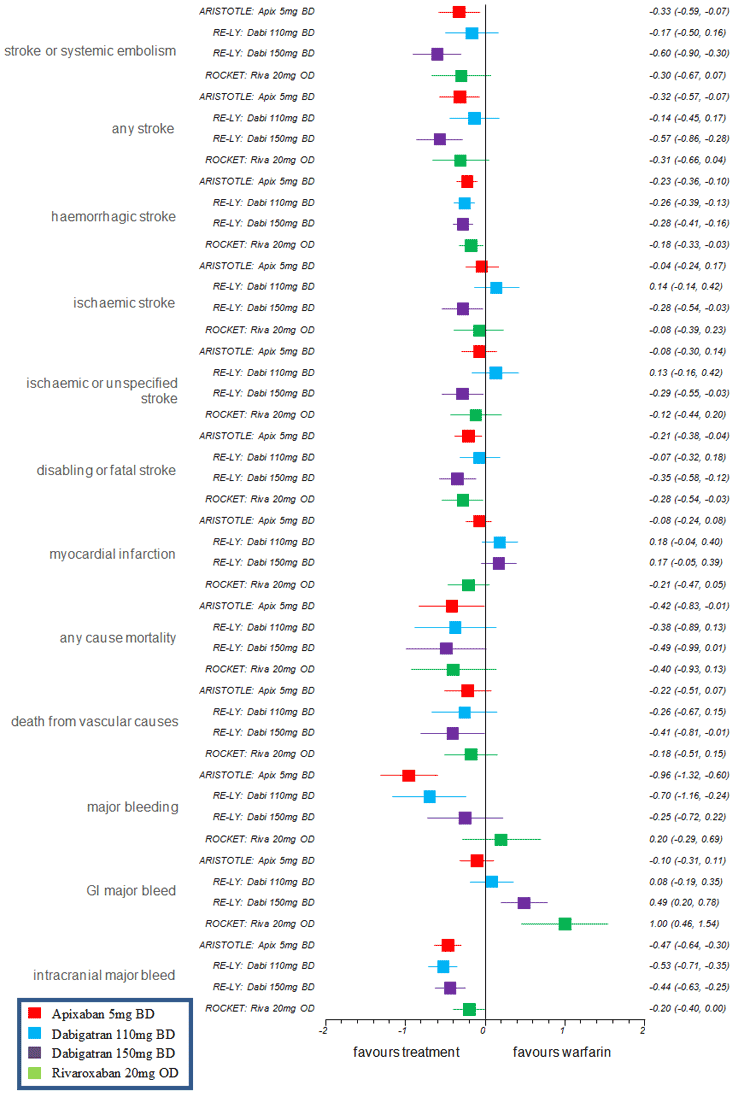
A summary of direct risk difference (RD) for all patient relevant outcomes of ARISTOTLE (apixaban 5 mg vs warfarin), RE-LY (dabigatran 110 mg vs warfarin), RE-LY (dabigatran 150 mg vs warfarin), ROCKET (rivaroxaban 20 mg vs warfarin) is shown in the figure below.
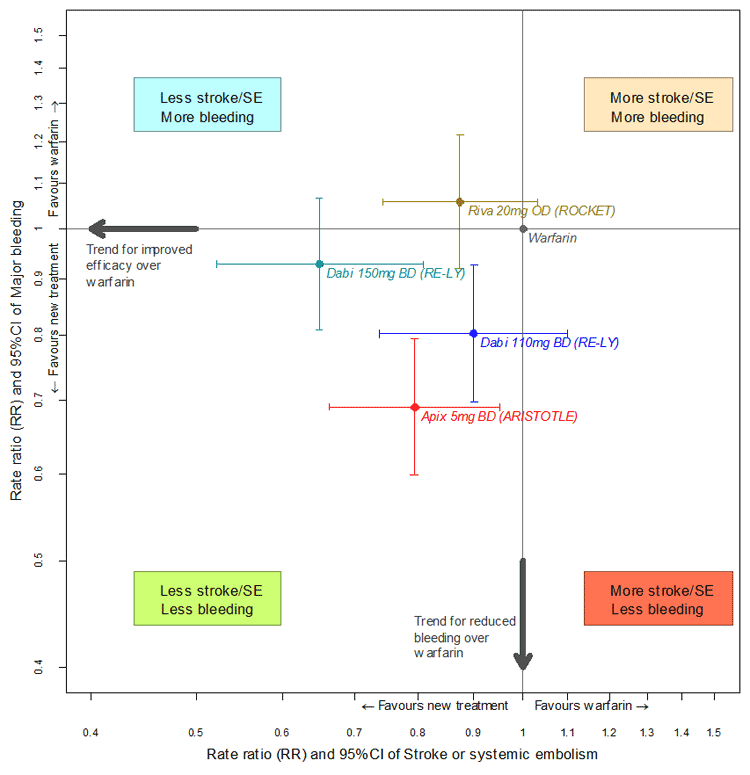
A novel oral anti-coagulant (NOAC) versus warfarin efficacy/safety map for the primary efficacy and safety outcomes from the trials is shown below.
The submission did not identify any additional safety concerns for either apixaban, dabigatran, rivaroxaban, warfarin or aspirin than what is already known in clinical practice or are documented in their respective draft/TGA approved Product Information or, in the case of dabigatran, recent FDA documentation.
For PBAC’s view, see Recommendation and Reasons.
9. Clinical Claim
The submission described that for NVAF (with at least one risk factor), apixaban is:
- superior to warfarin with respect to both efficacy and safety;
- superior to aspirin with respect to efficacy (stroke/SE) and has a comparable safety profile without any increases in bleeding events;
- non-inferior to dabigatran 150 mg and rivaroxaban with respect to efficacy (stroke/SE) and superior with respect to safety (major bleeding); and
- non-inferior to dabigatran 110 mg with respects to efficacy (stroke/SE), with a demonstrated trend towards lower major bleeding compared with dabigatran 110 mg.
For PBAC’s view, see Recommendations and Reasons.
10. Economic Analysis
Two modelled economic evaluations (cost-utility analysis/cost-effectiveness analyses) were presented in the submission comparing apixaban to:
- Current PBS comparators: 62.4% warfarin : 20.9% aspirin: 16.7% treatment (proportions derived from New Zealand dispensing data) and;
- Dabigatran 110 mg and 150 mg dosages (conducted separately in two models, results then collated assuming 50:50 use of each dosage form)
For the evaluation versus current PBS comparators, the modelled economic evaluation was based on the claim of superior efficacy and safety versus warfarin and superior efficacy and similar safety versus aspirin. The submission presented an ICER in the range of $15,000 - $45,000/QALY based on taking key stroke and bleeding outcomes from ARISTOTLE and AVERROES applied to a population similar to those enrolled in the ARISTOTLE trial and extrapolated to 20 years (from a median follow up of 1.8 years in the trial) and applying utility weights from Sullivan et al (2006).
For the evaluation versus dabigatran, the modelled economic evaluation was based on the claim of non-inferior efficacy and superior safety of apixaban versus dabigatran 110 mg and 150 mg. The submission presented an ICER in the range of $15,000 - $45,000/QALY based on taking key stroke and bleeding outcomes from ARISTOTLE and RE-LY applied to a population similar to those enrolled in the RE-LY trial and extrapolated to 20 years (from a medium follow up of 2.0 years in the trial) and applying utility weights from Sullivan et al (2006).
For PBAC’s view, see Recommendation and Reasons.
11. Estimated PBS Usage and Financial Implications
The submission estimated a net cost per year to the Government of greater than $100 million in Year 5.
For PBAC’s view, see Recommendations and Reasons.
12. Recommendation and Reasons
At the time of consideration, the PBAC noted that apixaban had not yet received TGA registration for the indication of “To reduce the risk of stroke, systemic embolism, and death in patients with non-valvular atrial fibrillation with at least one additional risk factor for stroke”. The PBAC further noted the ongoing nature of the Department’s review of novel oral anti-coagulants (NOAC).
The submission’s nominated comparators of dose-adjusted warfarin and aspirin, as well as dabigatran and rivaroxaban as potential future comparators, was considered appropriate by the PBAC.
The PBAC noted that the proposed restriction (with the exception of a history of coronary artery disease as a risk factor) is nearly identical to the restriction recommended for dabigatran in stroke and systemic embolism prevention in patients with non-valvular atrial fibrillation. The PBAC still considered that there is high potential for use of apixaban outside the restriction in patients with a low risk of stroke (i.e. a CHADS2 score of 0).
In evaluating the comparative effectiveness and safety of apixaban to warfarin, aspirin, dabigatran and rivaroxaban, the PBAC noted that in the randomised trial of apixaban versus dose adjusted warfarin (ARISTOTLE), apixaban 5 mg twice daily demonstrated a statistically significant reduction in the primary outcome of stroke or systemic embolism compared with warfarin (HR (95%CI): 0.79, (0.66, 0.95, p<0.001 for non-inferiority and p=0.01 for superiority, number needed to treat: 304). For the primary safety outcome of major bleeding, the PBAC noted that apixaban 5 mg reported a statistically significant lower risk of major bleeding when compared with warfarin (HR(95%CI): 0.69 (0.6, 0.8), number needed to treat: 105). The submission’s clinical claim that apixaban is superior to warfarin with respect to efficacy and safety was therefore accepted by the PBAC, noting however that the absolute effects were small.
In the one randomised trial of apixaban versus aspirin (AVERROES), the PBAC noted that apixaban significantly reduced the risk of stroke or systemic embolism when compared to aspirin treatment (HR (95%CI): 0.45 (0.32, 0.62, p<0.001). Although not reaching statistical significance, the safety results favoured aspirin for the primary safety outcome of major bleeding (HR (95%CI): 1.54 (0.96, 2.45, p=0.07). Other safety outcome measures also favoured aspirin and did reach statistical significance resulting in the PBAC accepting the submission’s clinical claim that apixaban has superior comparative effectiveness compared to aspirin but not accepting the clinical claim that apixaban has a comparable safety profile to aspirin.
For the comparison of apixaban to dabigatran and rivaroxaban, the submission presented one randomised trial comparing dabigatran (150 mg and 110 mg twice daily) versus dose adjusted open-label warfarin (RE-LY) and one randomised trial comparing rivaroxaban (20 mg daily, 15 mg daily in some patients) versus dose adjusted warfarin (ROCKET-AF). The PBAC noted that the indirect comparisons of apixaban 5 mg twice daily versus dabigatran 110 mg twice daily, dabigatran 150 mg twice daily and rivaroxaban 20 mg once daily (using warfarin as the common reference) did not produce any statistically significant differences between the treatments. The efficacy results of the indirect comparison favoured apixaban over dabigatran 110 mg and rivaroxaban but did not against dabigatran 150 mg in terms of the primary outcome of stroke/systemic embolism. However, some outcome measures of efficacy other than stroke/systemic embolism, particularly haemorrhagic stroke based on hazard ratio and relative risk, did not favour apixaban and so the PBAC was not convinced that apixaban’s efficacy is non-inferior to dabigatran or rivaroxaban. On balance, the PBAC considered that the true efficacy of apixaban is likely to lie somewhere between dabigatran 110 mg and dabigatran 150 mg, and similar to rivaroxaban. The PBAC therefore considered the clinical claim that apixaban’s efficacy is non-inferior to dabigatran 110 mg and rivaroxaban to be reasonable but did not accept the clinical claim that apixaban’s efficacy is non-inferior to dabigatran 150 mg.
In terms of apixaban’s comparative safety to dabigatran and rivaroxaban, the PBAC noted that the results suggested that apixaban’s safety was favourable compared to dabigatran 110 mg but not statistically significant, and, favourable and statistically significant compared to dabigatran 150 mg and rivaroxaban based on the primary safety outcome of major bleeding. In view of these results, the submission made the clinical claim that apixaban has superior safety compared to dabigatran and rivaroxaban. However, the safety outcome of intracranial bleeding suggested that apixaban is worse than dabigatran 110 mg and dabigatran 150 mg based on hazard ratios. Additionally, the PBAC noted that in the comparison with rivaroxaban, the subjects in the ROCKET-AF trial were a higher risk population, thereby potentially unfairly favouring apixaban. Taking into account the mixed safety results, the PBAC was not convinced that apixaban has superior safety to dabigatran and rivaroxaban, and therefore did not accept the clinical claim of superior safety for apixaban over dabigatran and rivaroxaban.
Based on the PBAC’s not accepting the clinical claim that apixaban is non-inferior to dabigatran and rivaroxaban in efficacy and superior to dabigatran and rivaroxaban in terms of safety, the PBAC did not consider the cost-utility/cost-effectiveness analysis versus dabigatran to be appropriate. Instead, based on the clinical trial results, the PBAC considered that a cost-minimisation analysis against dabigatran would be more appropriate.
The economic model was based on a 62.4% warfarin: 20.9% aspirin and 16.7% no treatment comparator split and the PBAC noted that the results of sensitivity analyses indicated that the model was moderately sensitive to the assumed composition of warfarin, aspirin and no treatment. When it was assumed that 100% warfarin will be replaced, the ICER increased to be in a range of $15,000 - $45,000 per QALY. The model was also sensitive to the assumed age related increase in risks, and when removed, the PBAC noted that the ICER increased by approximately $10,000/QALY.
The PBAC further noted that the economic analysis versus warfarin/aspirin was highly sensitive to CHADS2 disease severity. The economic analysis against warfarin was based on the ITT analysis of the main trial which encompassed a mix of patients with different disease severity – in the ARISTOTLE trial 34% of patients had a CHADS2 score of 1, 36% had CHADS2 score of 2, and 30% had a CHADS2 score of 3 and above. Therefore the cost-effectiveness of apixaban depends on the mix of patients treated in Australian clinical practice should apixaban be listed on the PBS. The PBAC noted that a recent paper by Lopes et al (2012) presented efficacy and safety results from the ARISTOTLE trial by CHADS2 score and that based on the sub-group of patients with a CHADS2 score of 1, as an illustration of how the ICER per QALY can vary significantly with disease severity, an incremental cost-effectiveness ratio in the range of $45,000 - $75,000 per QALY was generated by varying various parameters of the economic model. However, the PBAC did note that BEACH-SAND data suggest the mix of patients with CHADS2 scores in the ARISTOTLE trial is similar to that found in the Australian population. The PBAC further noted that the ICER could go up to a range of $75,000 - $105,000 per QALY gained if the upper 95% confidence interval is used for the hazard ratios for stroke-systemic embolism or all-cause mortality, ischaemic stroke, haemorrhagic stroke and major bleeding. This was considerably higher than the base case ICER in the range of $15,000 - $45,000 per QALY.
The PBAC considered the submission’s estimated financial implications for the PBS to be high and may be possibly underestimated if apixaban is used in patients with a less severe CHADS2 score of zero in clinical practice.
The PBAC therefore rejected the submission on the basis that the ICER for apixaban compared to warfarin/aspirin is uncertain and has the potential to be unacceptably high, and also because the PBAC did not accept the claim that apixaban has non-inferior efficacy and superior safety compared to dabigatran and rivaroxaban. The PBAC therefore did not accept the submission’s cost-effectiveness claim against dabigatran.
The PBAC noted the opportunity cost of listing apixaban for NVAF.
Recommendation:
Reject
13. Context for Decision
The PBAC helps decide whether and, if so, how medicines should be subsidised in Australia. It considers submissions in this context. A PBAC decision not to recommend listing or not to recommend changing a listing does not represent a final PBAC view about the merits of the medicine. A company can resubmit to the PBAC or seek independent review of the PBAC decision.
14. Sponsor’s Comment
The Sponsor is committed to working with the PBAC to resolve any perceived uncertainties to ensure access to apixaban in stroke prevention is delivered to Australian patients through PBS listing.




