Ivacaftor, tablet, 150 mg, Kalydeco® - July 2013
Public Summary Document
Product: Ivacaftor, tablet, 150mg, Kalydeco®
Sponsor: Vertex Pharmaceuticals
Date of PBAC Consideration: July 2013
1. Purpose of Application
The submission sought a Section 100 (Highly Specialised Drugs Program) listing or inclusion on the Life Saving Drugs Program (LSDP) for treatment of cystic fibrosis (CF) in patients aged six years and older who have a G551D mutation in the CFTR gene.
Highly Specialised Drugs are medicines for the treatment of chronic conditions, which, because of their clinical use or other special features, are restricted to supply to public and private hospitals having access to appropriate specialist facilities.
Life Saving Drugs Program:
Through the Life Saving Drugs Program (LSDP), the Australian Government provides subsidised access, for eligible patients, to expensive and potentially life saving drugs for very rare life-threatening conditions.
Before a drug is made available on the LSDP it must generally be accepted by the Pharmaceutical Benefits Advisory Committee as clinically necessary and effective, but not recommended for inclusion on the Pharmaceutical Benefits Scheme due to unacceptable cost-effectiveness.
This submission was considered under the TGA/PBAC parallel process. During the evaluation, the Clinical Evaluation Report, the TGA Delegates Overview and the ACPM outcome were available.
2. Background
The PBAC has not previously considered this product.
3. Registration Status
Ivacaftor was TGA registered on 9 July 2013 for the treatment of cystic fibrosis (CF) in patients aged 6 years and older who have G5526D mutation in the CFTR gene.
4. Listing Requested and PBAC’s View
The submission proposed the following 2 options:
Section 100 (Highly Specialised Drugs Program)
Authority required
OR
Life Saving Drugs Program
Treatment of cystic fibrosis in patients age six years or older who have a G551D mutation in the CFTR gene.
The PBAC noted that the submission was submitted under the TGA/PBAC parallel process; and that ivacaftor has now been registered by the TGA for the treatment of CF in patients age 6 years and older who have the G551D mutation in the CFTR gene.
The PBAC noted whilst post-lung transplant patients and patients with an FEV1 of less than 40% predicted were excluded from the presented trials (STRIVE and ENVISION), the requested listing did not explicitly exclude either patient group.
The Committee accepted the view of the clinical expert at the hearing that ivacaftor is likely to benefit post lung transplant patients because of its action on CFTR in other body systems, but considered the extent of benefit unknown, and potentially less, than in other CF patients with the G551D mutation.
The PBAC also noted that limited evidence is available for ivacaftor in more severe patients; i.e. those with a predicted FEV1% of less than 40%. The sponsor advised in its pre-sub-committee response is that 13 patients were randomised in the STRIVE and ENVISION trials with baseline FEV1 scores of < 40% (i.e. met the FEV1 criteria at screening, but fell below 40% between enrolment and randomisation). Of those patients, five were treated with ivacaftor. By week 24, four of the five patients had shown an improvement in lung function that was sustained in three of the four at week 48. The sponsor also advised that based on the ACFDR 2011, there are approximately 25 CF patients in Australia with the G551D mutation and a percent predicted FEV1 below 40%. Although there are limited data available that suggests treatment with ivacaftor does provide some benefit to these patients, as with post-lung transplant patients the impact on cost-effectiveness of treating patients in this category is unknown.
The PBAC considered it may be appropriate for continuation criteria to be included in the restriction, and noted the advice of the clinical expert at the hearing that normalisation of sweat chloride may be a useful early marker of a later more patient-relevant response.
5. Clinical Place for the Proposed Therapy
Cystic fibrosis (CF) is an autosomal recessive disease caused by mutations in the cystic fibrosis transmembrane regulator (CFTR) gene. The encoded protein, CFTR, is an epithelial chloride ion channel responsible for the regulation of salt and water absorption and secretion in multiple organ systems (lungs, pancreas, intestinal tract, biliary tract, sweat glands, and reproductive tract). Ivacaftor is a selective CFTR potentiator, which improves the function of the CFTR protein in patients with CF expressing the G551D mutation.
Ivacaftor would be used as add on to current therapy of CF, and has a different mechanism of action to the antibiotics, mucolytics and other treatments currently available through the PBS.
6. Comparator
The submission nominated best supportive care (BSC), consisting of usual respiratory, nutritional and rehabilitative support (e.g. mucolytics, osmotic agents, antibiotics, bronchodilatation, pancreatic enzymes, dietetic therapy, and chest physiotherapy) as the comparator.
The PBAC considered BSC was the appropriate comparator.
7. Clinical Trials
The submission presented the results of two trials, STRIVE and ENVISION. STRIVE was a randomised double blind trial comparing ivacaftor with BSC, in patients with CF involving a G551D mutation in the CFTR gene. The STRIVE trial (n=161, patients aged 12 years and over) both had a duration of 48 weeks. These trials were supported by evidence from an open-label extension trial of 48 weeks (PERSIST). All patients in STRIVE and ENVISON were offered the option of enrolling; 192 participated and data were provided for 185 patients out to 48 weeks for patients initially in STRIVE
One of these studies had been published at the time of submission, as follows:
|
Trial ID/ First author |
Protocol title/ Publication title |
Publication citation |
|---|---|---|
|
STRIVE
Ramsay BW et al |
A phase 3, randomised, double-blind, placebo-controlled, parallel-group study to evaluate the efficacy and safety of VX 770 in subjects with cystic fibrosis and the G551D mutation. |
New England Journal of Medicine 2011; 365:1663-1672
|
8. Results of Trials
The primary outcome reported in the STRIVE and ENVISON trials was absolute difference from baseline in percentage of predicted FEV1 to week 24. Secondary outcomes including mean absolute weight change, sweat chloride, time to pulmonary exacerbations, Cystic Fibrosis Questionnaire-revised (CFQ-R) respiratory domain score and in quality of life (QoL) as measured by the EQ-5D.
The results for the primary outcome of absolute change from baseline in percentage of predicted FEV1 to Week 24, and the corresponding change to Week 48, are presented below.
Trial results at 24 and 48 weeks
|
Trial |
|
Ivacaftor % predicted FEV1 |
Placebo % predicted FEV1 |
Absolute difference from baseline (95% CI) |
|---|---|---|---|---|
|
STRIVE |
Baseline |
63.46 (n=83) |
63.67 (n=78) |
|
|
|
Week 24 |
73.82 |
63.26 |
-10.58 (-12.59, -8.57) |
|
|
Week 48 |
73.53 |
62.89 |
-10.50 (-12.50, -8.50) |
The PBAC noted the results showed that there was an improvement in % predicted FEV1 of 10% from baseline in ivacaftor treated patients compared with BSC in both STRIVE and ENVISION at week 24 and week 48. The improvements in FEV1 % predicted were consistent with the submission’s nominated minimal clinically important difference (MCID) of 10%.
The PBAC further noted the results from the extension study, PERSIST, showed maintenance of the treatment effect on predicted FEV1% up to week 96. However, the PBAC also noted that the improvement in predicted FEV1% appeared to have declined at week 48 and 72 compared with week 24 in the ENVISION/PERSIST trial.
The PBAC also noted that treatment with ivacaftor was associated with improvements compared to BSC in the secondary outcome measures, including mean absolute change from baseline in weight, sweat chloride, time to pulmonary exacerbations, CFQ-R respiratory domain score and in QoL as measured by EQ-5D.The results for this last measure were used in the economic analysis in Part D of the submission.
With regard to comparative harms, the PBAC noted that in both trials, almost all patients in both the ivacaftor and placebo arms had at least one adverse event. Common adverse events (defined as occurring in at least 10% of patients in at least one arm of one trial) were ‘CF lung’, coughs, upper respiratory tract infections, headaches, haemoptysis, productive cough, arthralgia, sinusitis, abdominal pain, oropharyngeal pain, nasopharyngitis, vomiting, pyrexia, rales and rhinorrhoea. In STRIVE, serious adverse events occurred more frequently in the placebo group than in the ivacaftor group (42.3% vs. 24.1%, p=0.013), but this was not observed in ENVISION.
9. Clinical Claim
The submission described ivacaftor as superior in terms of comparative effectiveness and equivalent in terms of comparative safety over BSC.
The PBAC considered that the claim of superiority was supported in terms of the effect on the surrogate outcomes reported in the trials at 24 at 48 weeks, including % predicted FEV1, weight gain and QoL. However, the short term follow-up period means that there is no evidence directly from the trials that the change in FEV1 will translate into long term survival benefits.
The PBAC agreed that the claim of equivalent safety was supported in the short term but noted the long term safety of ivacaftor is unknown. This is a concern given that ivacaftor would be initiated from childhood as a lifelong treatment.
10. Economic Analysis
The submission presented a stepped economic evaluation based on the superiority claim for comparative benefit. The model uses the 48 week FEV1 outcome data reported in the STRIVE and ENVISON trials, applied to a CF population with a G551D mutation and extrapolates it to 74 years (patients are modelled from the age of 6 to 80).
The base case ICER was greater than $200,000 per QALY.
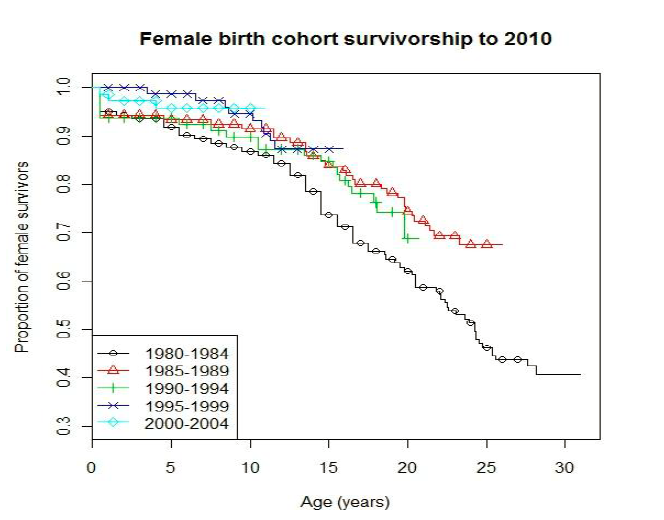
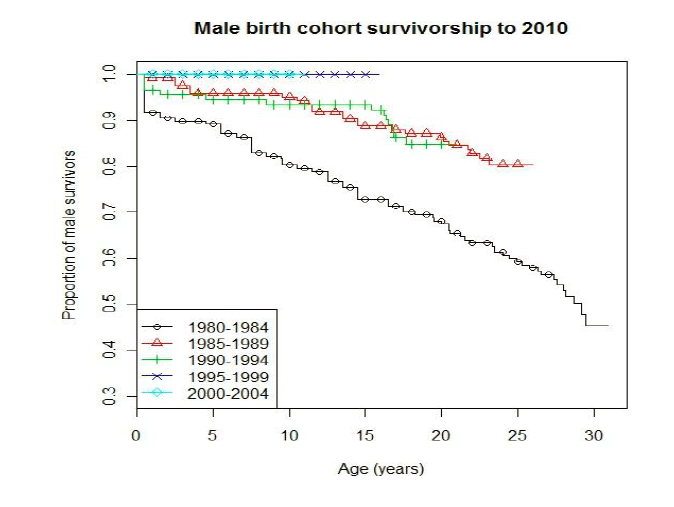
The submission presented the following figures to show survival in the CF population by birth cohort (1980-2004) in Ireland. The survival curves from the 3 most recent birth cohorts (1990-1994, 1995-1999, 2000-2004) were fitted to a Weibull function to predict survival for current CF patients. The Pre Sub-Committee Response (PSCR) stated that the fit of four parametric distributions used to extrapolate survival in CF beyond the observed data were assessed by Jackson et al. (2011), who reported that the Weibull distribution best fitted the observed data.
The Survival in total CF population, by birth cohort 1980 - 2004, Republic of Ireland is shown in the following two figures:
The Republic of Ireland survival curves fitted to Weibull function – Total CF population is shown in the following figure:
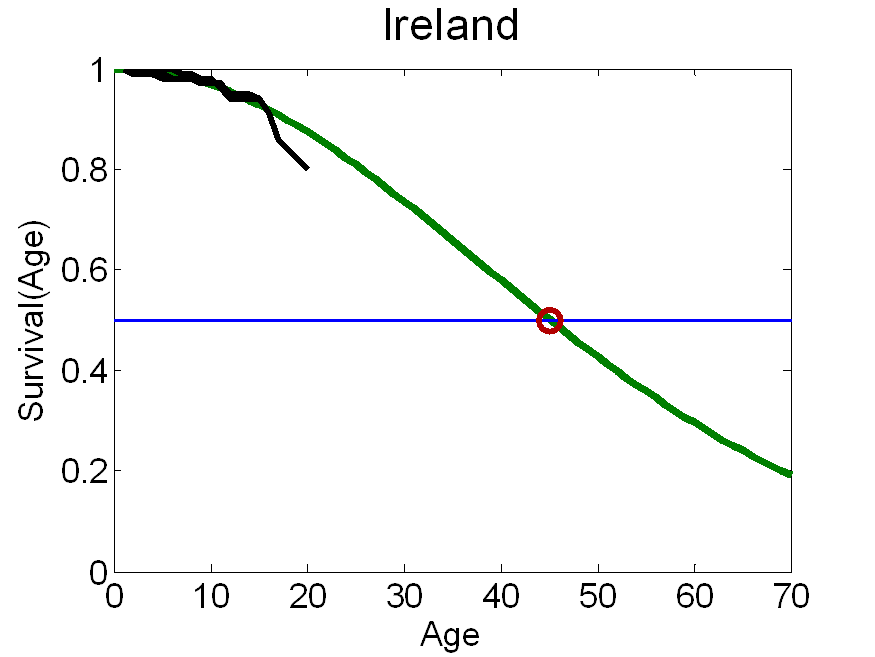
Note: the green line represents the modelled survival curve. The three black lines are the survival curves that were fitted to the Weibull function. The red dot represents the estimate for median survival (45.1 years) based on a patient-level actuarial survival analysis (Jackson et al, 2011). Formula for Weibull: S(a) = exp(-0.00044043*(a-1)^1.9441), where a is the age of an individual. “a-1” because we have normalised survival to 100% in year one. See (Klein and Moeschberger 2003) “Survival Analysis” table 2.2.
The PBAC considered that the extrapolation of survival for patients not treated with ivacaftor was not considered reliable. The survival data presented show an improvement in survival for the most recent 2000-2004 cohort compared to the 1990-1994 cohort (particularly in males), so including the earlier cohort in calculations of the predicted survival beyond 15 years is likely to underestimate current CF survival receiving BSC and thus overestimate any gains modelled from treatment with ivacaftor.
The submission’s approach to estimating the mortality gain from ivacaftor requires a number of steps, each of which introduces considerable uncertainty. Overall the accumulation of these steps means that the final estimate of mortality gain is unlikely to be reliable. The steps are:
1) FEV1 % predicted (and other factors that lead to death such as weight loss and pulmonary exacerbations) is extrapolated from the 48 week trial data out to a lifetime duration. In the base case, patients in the ivacaftor arm experience no decline in %FEV1 over time, whereas patients receiving supportive care alone experience an annual decline.
2) The Liou et al formula, derived from the Liou study (2001), an Italian observational study of a cohort, is used to predict risk of death based on the extrapolated clinical factors (from step 1);
3) The difference is risk of death between the ivacaftor and supportive care arms is estimated (from the results of step 2). This risk difference is then applied to the survival curve for current CF patients (generated from the Irish data above) to give an estimated survival curve for an ivacaftor treated patient.
Concerns about the extrapolation from change in predicted FEV1% to mortality include:
- The estimation of current survival is based on historical international data and does not take account of observed improvements in survival of the CF population;
- The use of the Liou et al. data assumes the effect of each improved clinical measure on survival is additive. The assumptions that the effects on survival are causal and additive may not be appropriate; and
- The effect of ivacaftor on FEV1 and BSC is extrapolated beyond the 48 weeks of trial data in STRIVE and ENVISION to a lifetime time horizon. As noted above, there are no longer-term follow-up data to support this extrapolation.
The overall effect of these assumptions is that the effect of ivacaftor on survival is likely to be overestimated.
Additional translation issues include:
- The comparison of patient characteristics between the trials and the Australian population is limited to age, gender and FEV1. Thus, applicability has not been established;
- The utility weights used in the economic analysis in Section D are based on trial data. The conversion of the raw data to utility weights could not be replicated, and the utility weights stratified by FEV1 score (used in Section D) are not provided in the Clinical Study Reports. Overall, patients in the trials had high utility values, which may reflect that the EQ-5D is a relatively poor instrument at resolving between small but important differences in utility and may not be the appropriate multi-attribute utility instrument (MAUI) for capturing the impact of cystic fibrosis on a patient’s overall quality of life.
The findings of van Gool et al. (2013) are used to estimate the cost of BSC in both arms of the economic evaluation.
11. Estimated PBS Usage and Financial Implications
The likely number of patients per year was estimated in the submission to be less than 10,000 per year in Year 5, at an estimated net cost per year to the PBS of $60 million - $100 million in Year 5.
The PBAC noted that there is strong consumer support for ivacaftor and considered that it would likely to be universally used in CF patients with G551D mutation, and therefore the submission’s estimate of the number of patients treated was considered reasonable.
However, the PBAC also considered that there would be a high potential for leakage into other subgroups of CF patients given the biological plausibility that ivacaftor will be effective in some of those subgroups (albeit to a likely smaller extent) and the clinical trials including its use in these populations are ongoing.
12. Recommendation and Reasons
The PBAC considered the submission, the commentary, the Economics Subcommittee (ESC) advice, and the sponsor’s pre-subcommittee response, pre-PBAC response and hearing.
The PBAC recognised the potential clinical value of ivacaftor in the treatment of cystic fibrosis (CF) in patients aged six years or older who have a G551D mutation, and agreed that the demonstrated 10% improvement in forced expiratory volume in one second (FEV1) over a period of up to 2 years is clinically significant and important. The Committee recognised that ivacaftor represents an advance over currently subsidised treatments for cystic fibrosis.
The PBAC acknowledged the many consumer comments received in relation to the submission, both from people living with the condition and on behalf of patients and their carers. The PBAC also acknowledged the correspondence from The Thoracic Society of Australia & New Zealand and The CF Foundation. The Committee recognised the strong support for subsidised access to ivacaftor.
The PBAC formed the view that the Pharmaceutical Benefits Scheme (PBS) is the most appropriate mechanism for subsidising ivacaftor for Australian patients, but that, at the price proposed in the submission, the cost per Quality Adjusted Life Year (QALY) is too high and too uncertain. The PBAC noted that it may be possible to reduce the dose of ivacaftor in clinical practice by co-administering ivacaftor with a strong CYP3A inhibitor, but that even with a dose reduction, the cost per QALY would remain too high. The PBAC decided to defer making a recommendation to allow the sponsor the opportunity to consider the Committee’s views and to submit a new price proposal for PBS listing.
Having deferred the request for PBS listing, the PBAC did not proceed to consider ivacaftor for listing on the LSDP, as a prerequisite for a recommendation for such a listing is that the drug be rejected for PBS listing.
Further details on the PBAC’s recommendations follow:
The PBS as the appropriate mechanism for subsidy of ivacaftor:
The PBAC noted that currently around 3000 Australians have cystic fibrosis, the most common life-shortening, autosomal recessive disease affecting people of European ancestry. Around 8.6%, or 250, of these patients are estimated to have the G551D mutation of the CFTR gene, making ivacaftor a highly targeted therapy with a relatively small patient population.
The PBAC noted that a number of clinical trials of ivacaftor in other subgroups of patients with CF are underway, making it plausible that the group of patients who will potentially benefit from this treatment will increase over time.
The Committee also noted that molecularly targeted therapies are becoming increasingly common, with a number of such therapies being considered for, or added to, the PBS over the past few years. Whilst some molecularly targeted therapies are PBS subsidised for larger patient populations than will be treated with ivacaftor (eg. cetuximab for colorectal cancer), others are subsidised through the PBS for even rarer conditions than CF with a G155D mutation. For example, imatinib is PBS subsidised for dermatofibrosarcoma protuberans (DFSP), hypereosinophilic syndrome or chronic eosinophilic leukaemia (HES/CEL), myelodysplastic syndromes or myeloproliferative disorders (MDS/MPD), and aggressive systemic mastocytosis (ASM).
Lastly, the PBAC noted that the PBS is the mechanism through which the Australian Government provides subsidised access to the medicines currently used by CF patients living in the community, including mucolytics, antibiotics and pancreatic enzyme preparations.
Overall, the Committee considered the PBS the most appropriate mechanism for subsidising ivacaftor for Australian patients.
Although the PBAC did not proceed to a detailed consideration of ivacaftor against the LSDP criteria, the Committee expressed its concern that the LSDP is not suitable as a means of subsidising access to a treatment for the most common autosomal recessive disease affecting Australians of European ancestry. A total of 215 patients with one of 7 separate disorders were treated with one of the 9 drugs subsidised through the LSDP in 2011-12[1]: this is less than the number of G155D CF patients that the submission estimates will be treated with ivacaftor in the first year of subsidy.
Cost-effectiveness of ivacaftor
The PBAC considered that at the requested price, the requested listing for ivacaftor is not sufficiently cost effective to enable PBS listing. Additionally the estimated cost per QALY is likely to be underestimated.
The PBAC agreed with the major areas of uncertainty identified by its Economics Sub-Committee (ESC), which include:
- the extrapolation of survival for patients not treated with ivacaftor is not reliable. The data presented clearly show an improvement in survival for the most recent 2000-2004 cohort compared to the 1990-1994 cohort (particularly in males), so including the earlier cohort in the predicted survival beyond 15 years is likely to underestimate current CF survival and so overestimate the survival gain attributed to ivacaftor;
- the submission’s approach to estimating the mortality gain from ivacaftor requires a number of steps, each of which introduces considerable uncertainty. The magnitude of the true survival benefit with ivacaftor cannot be accurately quantified given the effect of ivacaftor and BSC on FEV1 is extrapolated from 48 weeks to 74 years. The effect of intermediate outcomes on survival is estimated using Liou et al. (2001), which is based on observed associations between clinical parameters and survival from the 1990s. The study does not demonstrate these relationships are causal, although this is implicitly assumed in the submission’s approach, and must be interpreted in the context of changes in treatment over the past two decades. The patient-level data upon which the Liou study are based are now quite old and the estimates may not be replicated with more recent cohorts;
- the EQ-5D is likely to be an insensitive measure in the CF context. Overall, patients in the trial had high utility values, which may reflect that the EQ-5D is a relatively poor instrument at resolving between small but important differences in utility and may not be the appropriate multi-attribute utility instrument (MAUI) for capturing the impact of cystic fibrosis on a patient’s overall quality of life. In addition, the submission’s conversion of the raw data to utility weights could not be replicated, and the utility weights by FEV1 score (which are the ones used in the economic analysis in Section D) were not provided in the Clinical Study Report.
The PBAC considered that the true ICER based on survival was likely to be higher than the model base case because these assumptions in the model favour treatment with ivacaftor.
However, the PBAC also agreed that the ICER derived from the model presented in the submission did not capture all of the potential health benefits from treatment with ivacaftor and that some potential and relatively small cost offsets had not been accounted for in the model, for instance from hospitalisations avoided or from the potential reduction in use of other medicines.
Overall, the cost of the drug and the estimation of the survival benefit were considered the key issues by PBAC, as these are the drivers of the model and the resulting high and uncertain cost-effectiveness ratio.
Alternative dosing regimens for ivacaftor
The PBAC noted that the Dosage and Administration section of the Australian Product Information (PI) for ivacaftor states that, when co-administered with strong inhibitors of CYP3A (e.g., ketoconazole, itraconazole, posaconazole, voriconazole, telithromycin and clarithromycin), ivacaftor should be administered at a dose of 150 mg twice a week.
The Committee considered that, were such a dosing regimen able to be used in place of the 150 mg twice daily dosing regimen upon which the submission was based, the cost of the drug would be significantly lower. Given the high cost and high clinical need for this drug, the PBAC requested the sponsor consider, and comment on, the suitability of this PI recommended alternate dosing regimen for routine use.
Outcome:
Defer
13. Context for Decision
The PBAC helps decide whether and, if so, how medicines should be subsidised in Australia. It considers submissions in this context. A PBAC decision not to recommend listing or not to recommend changing a listing does not represent a final PBAC view about the merits of the medicine. A company can resubmit to the PBAC or seek independent review of the PBAC decision.
14. Sponsor’s Comment
The sponsor had no comment.
[1] Department of Health and Ageing Annual Report 2011-12




