Infliximab, powder for intravenous infusion, 100mg, Remicade® - November 2013
Public Summary Document
Product: Infliximab, powder for intravenous infusion, 100mg, Remicade®
Sponsor: Janssen-Cilag Australia Pty Ltd
Date of PBAC Consideration: November 2013
1. Purpose of Application
The major re-submission sought to extend the current Section 100 Highly Specialised Drugs Program Public and Private Hospital Authority required listings to include treatment of acute severe ulcerative colitis not responding to IV corticosteroids in a patient aged 6 years or older who meets certain criteria.
Highly Specialised Drugs are medicines for the treatment of chronic conditions, which, because of their clinical use or other special features, are restricted to supply to public and private hospitals having access to appropriate specialist facilities.
2. Background
This was the second submission for infliximab considered by the PBAC for acute severe ulcerative colitis.
In March 2013, the PBAC rejected an application on the basis that:
- the comparator should have also included cyclosporin;
- the evidence base for infliximab’s efficacy was limited to a small number of trials in the acute severe ulcerative colitis setting; and
- there was uncertainty in the economic modelling that potentially resulted in a high and unacceptable ICER.
The public summary document is available at the PBS website.
3. Registration Status
Infliximab is TGA registered for use in adults and in children and adolescents (6 to 17 years) for the treatment of moderately severe to severe active ulcerative colitis in patients who have had an inadequate response to conventional therapy (May 2012).
4. Listing Requested and PBAC’s View
Section 100 (Highly Specialised Drugs Program)
Authority Required (Streamline) for Public Hospitals
Authority Required for Private Hospitals
Initial PBS-subsidised treatment by a gastroenterologist or consultant physician as specified in the NOTE below, of a patient aged 6 years or greater with acute severe ulcerative colitis who satisfies the following criteria:
(a) an adult who has acute severe ulcerative colitis as defined by the presence of more than 6 bloody stools per day, plus at least one of the following signs:
- temperature more than 37.5°C
- pulse rate more than 90 / minute
- haemoglobin less than 105 g / L
- Erythrocyte sedimentation rate greater than 30 mm/h.
OR
(b) a child aged six years or more who has severe ulcerative colitis as defined by a Paediatric Ulcerative Colitis Activity Index (PUCAI) ≥ 65
With the diagnosis confirmed by a gastroenterologist or a consultant physician as specified in the NOTE below.
AND
(c) who has failed to achieve an adequate response to at least 72 hours intravenous corticosteroids.
For adults, failure to achieve an adequate response is defined by the Oxford criteria where:
• If assessed on day 3, patients pass 8 or more stools per day or 3 or more stools per day with a CRP > 45 mg / L
• If assessed on Day 7 patients pass 3 or more stools per day with visible blood.
For children aged 6 to 15 years failure to achieve an adequate response means PUCAI score >45 at 72 hours. Before administering infliximab the treating clinician must have consulted with a paediatric gastroenterologist or with an institution experienced in performance of paediatric colectomy.
Note:
Prescribers must be gastroenterologists (code 87), consultant physicians [internal medicine specialising in gastroenterology (code 81)] or consultant physicians [general medicine specialising in gastroenterology (code 82)].
5. Clinical Place for the Proposed Therapy
Patients with acute severe episodes of ulcerative colitis are usually admitted to hospital for monitoring and IV corticosteroids e.g. hydrocortisone 400 mg/day or methylprednisolone 60 mg/day. Patients who respond to corticosteroids will have their steroids tapered and be offered maintenance therapy with 5-aminosalicylates or immunomodulators e.g. 6-mercaptopurine. Patients who fail to respond to IV corticosteroids in 3-7 days can be offered emergency surgery (colectomy), best supportive care (BSC) including continuing IV corticosteroids, infliximab or cyclosporin. Expert advice quoted in the re-submission considered that currently 10-20% of the patients are being treated with cyclosporin. Responding patients will have their steroids tapered orally and maintained on 5-aminosalicylates or immunomodulators whereas those who fail to respond will be considered for surgery (colectomy).
Patients will be in a hospital while receiving the first infliximab infusion. The infliximab item on Section 100 is not available as a PBS benefit for in-patients of a private or public hospital. As such, accessibility will be dependent on inclusion within a public hospital formulary (public hospital patients) or private health insurance or individual patient’s capacity to pay for the first infusion (private hospital patients). Various hospitals around the country have infliximab on their formulary and the re-submission argued that this may result in inequity with availability not uniformly available within Australia.
The PBAC agreed with the Economic Sub-committee (ESC) that PBS listing is not a requirement for inclusion of a drug on hospital formularies. The PBAC also noted that infliximab costs for the initial dose will be borne by the hospital (through State Government, third party funder or self-funded reimbursement). The PBAC noted the submission’s claim that a PBS listing for the two additional doses will signal to hospitals that infliximab is a cost-effective intervention and will stimulate formulary listings.
6. Comparator
The re-submission maintained BSC as the main comparator, and added cyclosporin to the analysis as a ‘minor’ comparator at the request of the PBAC.
The PBAC agreed that cyclosporin is an appropriate comparator.
7. Clinical Trials
For the comparison of infliximab versus BSC, the re-submission was based on three randomised trials: Jarnerot et al., 2005, Sands et al., 2001 and Florholmen et al., 2011, including a total of 82 patients who were followed up for 7 to 90 days, with extension data up to three years. The PBAC noted that these were the same data as presented in the previous submission. Details have been previously reported in the March 2013 PSD.
For the comparison of infliximab versus cyclosporin, the re-submission presented one randomised trial (Laharie 2012) and a prospective non-randomised Australian study (Croft 2012). Details of the trials are presented in the table below.
|
Trial |
Protocol title/ Publication title |
Publication citation |
|
|---|---|---|---|
|
Infliximab vs. cyclosporin |
|||
|
Laharie 2012 |
ClinicalTrials.Gov identifier: NCT 00542152.Study Comparing Cyclosporin With Infliximab in Steroid-refractory Severe Attacks of Ulcerative Colitis.
Laharie et al. Cyclosporin Versus Infliximab in Severe Acute Ulcerative Colitis Refractory to Intravenous Steroids: A Randomized Trial
Laharie et al. Ciclosporin versus infliximab in patients with severe ulcerative colitis refractory to intravenous steroids: a parallel, open-label randomised controlled trial. |
Gastroenterology 2011 14) Abstract 619
Lancet 2012, 1;380(9857):1909-15 |
|
|
Croft 2013 |
Croft et al. Outcomes of salvage therapy for steroid-refractory acute severe ulcerative colitis: ciclosporin vs. infliximab. |
Alimentary Pharmacology & Therapeutics, 2013, 38 (3) 294-302 |
|
The PBAC noted that the Laharie trial (n=115, patients followed for 98 days) was identified in the previous submission as the ‘CYSIF’ trial. The PBAC further noted that it was not included as pivotal trial evidence in the previous submission, as the previous submission only considered the comparison with BSC to be relevant.
The Croft study data is a post-hoc analysis of prospective data of 98 patients in an Australian hospital over 10 years. Data collection began in 1999 on patients presenting at the hospital with acute severe ulcerative colitis. Patients who subsequently failed hydrocortisone therapy self-selected either colectomy or rescue therapy (either cyclosporin or infliximab). Notably, treatment with infliximab was not available until 2001, and cyclosporin therapy changed in 2003 from one infusion of 4 mg/kg with no subsequent oral therapy, to 2 mg/kg followed by three month’s oral therapy. Patients who self-selected colectomy after failing hydrocortisone therapy were excluded from the analysis. The PBAC noted that the risk of bias for the Croft study is high as the study is non-randomised.
8. Results of Trials
The results for the comparison of infliximab versus BSC have been previously reported in the March 2013 PSD.
The following table summarises the relative effect, event rates and risk difference from the Laharie (2012) and Croft (2012) trials.
Infliximab versus cyclosporin in acute severe ulcerative colitis
|
Outcome Primary |
Number of participants (studies) |
Relative effect from trial(s) Relative Risk |
Cyclosporin event rate per 100 patients per 3 months |
Infliximab event rate per 100 patients per 3 months |
Increment Risk Difference |
|---|---|---|---|---|---|
|
Benefits |
|||||
|
Colectomies at 3 months |
|
|
|
|
|
|
Laharie (RCT) |
115 |
1.2 (0.6, 2.6) |
17 per 100 |
21 per 100 |
4% (-11%, 18%) |
|
Croft (Cohort study) |
80 |
0.5 (0.3, 1.0) |
47 per 100 |
24 per 100 |
-22% (-43%, -2%) |
|
Harms |
|||||
|
Laharie |
|
|
|
|
|
|
Severe Infections |
115 |
1.27 (0.4, 4.5) |
7 per 100 |
9 per 100 |
2% (-8%, 12%) |
|
Worsening of ulcerative colitis |
115 |
2.4 (0.7, 8.7) |
5 per 100 |
12 per 100 |
7% (-3%, 17%) |
|
Hepatic event |
115 |
.. |
0 per 100 |
7 per 100 |
7% (0%, 14%) |
|
Croft |
|
|
|
|
|
|
Seizure |
80 |
.. |
5 per 100 |
0 per 100 |
-5% (-11%, 2%) |
The results of patient relevant outcomes from the Laharie (2012) and Croft (2013) trials are presented in the table below.
|
|
IFX n/N (%) |
CSP n/N (%) |
p-value |
ORa (95% CI) |
RD (95% CI) |
NNT (95% CI) |
|---|---|---|---|---|---|---|
|
Laharie 2012 (randomised trial) |
||||||
|
Treatment Failure, day 98 |
31/57 (54%) |
35/58 (60%) |
0.52 |
0.8 (0.4, 1.8) |
-6% (-24.0, 12%) |
NE |
|
Clinical response, day 7 |
48/57 (84%) |
50/58 (86%) |
0.76 |
0.9 (0.3, 2.7) |
-2% (-15%, 11%) |
NE |
|
Colectomy, day 98 |
12/57 (21%) |
10/58 (17%) |
0.60 |
1.3 (0.5, 3.7) |
-4% (-18%, 11%) |
NE |
|
Croft 2013 (non-randomised study) |
||||||
|
Colectomy-free survival at discharge |
32/38 (84%) |
24/43 (56%) |
0.006 |
0.2 (0.1, 0.6) |
28% (10%, 47%) |
4 (2, 10) |
|
Colectomy-free survival at three months |
28/37 (76%) |
23/43 (53%) |
0.04 |
0.4 (0.1, 1.2) |
22% (2%, 43%) |
5 (2, 53) |
|
Colectomy-free survival at twelve months |
24/37 (65%) |
18/43 (42%) |
0.04 |
0.4c (0.1, 1.3) |
23% (2%, 44%) |
4 (2, 59) |
INF = infliximab; CSP = cyclosporin; OR = odds ratio; ARD = absolute risk difference; NNT = number needed to treat; NE = not estimable; bold = statistically significant
aValue below 1.0 indicates that infliximab is more effective than cyclosporin
The PBAC noted that the Laharie trial did not show statistically significant differences in patient-relevant outcomes between the trial arms. The Croft study reported that patients treated with infliximab had statistically significantly improved clinical outcomes to cyclosporin-treated patients. However, the PBAC noted that the Croft study is non-randomised and has a high risk of bias, leading to a likely overestimate of the effect of infliximab.
The PBAC considered that based on the Laharie 2012 trial, infliximab could be considered non-inferior to cyclosporin in terms of comparative effectiveness.
The PBAC noted that the ESC did not accept the re-submission’s claim of superior clinical efficacy of three infusions as compared to a single infusion of infliximab, based on a naïve comparison of the infliximab arms in Jarnerot (2005), Croft (2013) and Laharie (2012) trials presented in the figure below. The PBAC further noted the ESC agreed that the relative efficacy of three infliximab infusion was highly uncertain because the potential benefits from additional infusions were difficult to interpret and were not supported by the evidence provided.
Comparison of the infliximab arms in Jarnerot (2005), Croft (2013) and Laharie (2012) trials
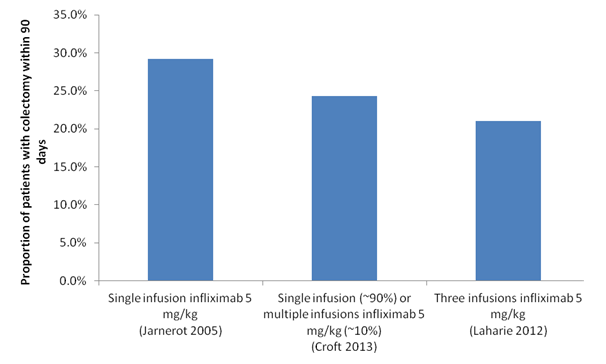
The PBAC noted that there was no comparative evidence of the efficacy of multiple infusions of infliximab compared with a single infusion. Based on the naïve indirect comparison of the three trials above, the PBAC noted that:
- The three data points were insufficient to demonstrate a trend, and appropriate analysis would require further statistical testing to demonstrate any significant treatment effect from additional infusions.
- The graph showed the efficacy of infliximab infusions in individual trials. However, it did not show clinical or statistical evidence of superior efficacy from additional infusions of infliximab between these trials.
- Four patients in the Croft 2013 study had an unknown number of additional infusions, and these were reported at 12 months after admission. This was outside the three month scope of the economic evaluation presented in the re-submission. As such, the graph illustrated the outcomes of a single infusion only of infliximab within 90 days for Jarnerot 2005 and Croft 2013, and three infusions for Laharie 2012.
However, the PBAC also noted the advice of the Gastroenterological Society of Australia (GESA) in relation to the use of infliximab for acute severe ulcerative colitis. GESA advised that because of previous data showing high antigenicity with episodic or single dose therapy, all clinicians endorse that full induction therapy (3 doses) should be given. Moreover, this allows disease control for a sufficient duration for an immunomodulator to become effective. On the strength of this advice, the PBAC accepted that a treatment course consisting of three doses was appropriate.
The PBAC noted that infliximab is associated with an increased risk of opportunistic infection as well as infusion reactions, fever and rash. Malignancy is rare with the use of infliximab although a causal relationship is unclear. The re-submission stated that there is an Australian Risk Management Plan (RMP), which is based upon the European Risk Management Plan, for infliximab. The Sponsor’s risk management plan covered the communication of general safety information as well as the following specific areas:
- Risk of tuberculosis and other infections
- Safety in paediatric Crohn’s disease and paediatric ulcerative colitis.
The PBAC noted that the short term and common infliximab adverse events reported in literature, e.g. infusion related reactions, are similar to those observed in the clinical trials and are mild to moderate in severity.
In comparison, the PBAC noted that cyclosporin must be monitored closely to manage its toxicity levels. The risk of minor side-effects with cyclosporin ranged from 31% to 51% and included tremor, paraesthesia, malaise, headache, abnormal liver function, gingival hyperplasia and hirsutism. Major complications carry a risk of up to 17% and include renal, infectious and neurotoxic effects such as seizures and anaphylaxis with the intravenous preparation.
9. Clinical Claim
The re-submission described infliximab as having superior efficacy and a different but comparable safety profile compared with placebo (BSC) for adult and paediatric patients with acute severe ulcerative colitis not responding to IV corticosteroids. The PBAC previously considered that the clinical claim was reasonable for adult patients.
The re-submission described infliximab as superior in terms of comparative efficacy and different and superior in terms of comparative safety compared with cyclosporin.
The PBAC agreed with the ESC that the clinical claim of superior efficacy of infliximab over cyclosporin was not reasonable as the claim was based on low quality data with a high risk of bias (non-randomised post-hoc analyses of a cohort study Croft 2013) and was not supported by evidence from a randomised controlled trial (Laharie 2012).
The PBAC considered that infliximab is no worse than cyclosporin in terms of comparative efficacy and comparative safety.
10. Economic Analysis
The re-submission presented a 90-day decision tree model based cost effectiveness analysis (incremental cost/surgery avoided) comparing infliximab with best supportive care based on a claim of superior efficacy. The efficacy results of the Jarnerot 2005 trial (single infusion) were applied to the economic evaluation and included economic savings from colectomies avoided with infliximab.
The PBAC noted that the cost-effectiveness analysis included infusion site reactions cost estimates, updated usual care maintenance drug therapy, hospitalisation admission and colectomy-related surgery costs, and appropriately newly includes infliximab administration and ostomy care costs. The 90-day decision tree model consisted of three health states: acute severe ulcerative colitis (hospitalised and not responding to IV corticosteroids), post-treatment ulcerative colitis and post-surgery (colectomy).
The re-submission estimated that infliximab compared with best supportive care would be the dominant strategy as infliximab will result in fewer colectomies and a cost saving over the 90-day time period, due to savings from colectomies avoided. The PBAC noted that there remained uncertainty around the magnitude of the number of colectomies avoided, as the Jarnerot trial had a high risk of bias. The PBAC also noted that the economic model is highly sensitive to the cost of colectomy and the probability of surgery, with all analyses resulting in infliximab being the dominant therapy, with one exception in which the lower 95% confidence interval for infliximab from the Jarnerot trial is combined with a lower cost of colectomy.
The re-submission also presented a cost-minimisation analysis based on the claim that infliximab is no worse in terms of comparative efficacy and different but comparable tolerability and safety profile compared to cyclosporin with regular monitoring and prophylactic treatment for Pneumocystis jirovecii. Due to this different claim in comparative efficacy (compared to the clinical claim), the re-submission presented a 98-day cost minimisation analysis comparing three infusions of infliximab with cyclosporin. The analysis was based on the trial outcomes of the Laharie 2012 study, and advice received from the PBAC. The PBAC noted that there were no cost offsets included in the analysis.
The re-submission stated the equi-effective doses of infliximab and cyclosporin as based on the planned dosage schedule in the Laharie 2012 trial are infliximab: 3 infusions at days 0, 14 and 42 of 5 mg/kg and cyclosporin: 7 days 2 mg/kg followed by 90 days of oral 4 mg/kg.
The PBAC noted that the steady state dose of cyclosporin in the Laharie trial was not reported. The PBAC also noted that the cost of hospitalisation per day, cyclosporin treatment, cyclosporin monitoring, infliximab infusion and infusion site reaction costs were included in the analysis.
The PBAC noted that the base case analysis at the proposed listing price indicated savings per patient of less than $10,000. The re-submission implied a price per infliximab infusion that was higher than the proposed price.
The PBAC further noted that the incremental length of stay was driving the modelled cost savings with infliximab, having assumed a 9-day incremental stay associated with cyclosporin based upon a retrospective study of 38 patients in New Zealand (Dean 2011). The PBAC agreed with the ESC advice that 6 days may be a more reasonable assumption of hospital days saved, based on the Lowenberg (2013) abstract of a retrospective study conducted in the Netherlands (n=42) which showed a significantly shorter length of stay in hospital in patients treated with infliximab as compared with cyclosporin (4.5 days v 10.3 days, p=<0.0001).
Overall, the PBAC agreed that the cost-minimisation analysis against cyclosporin was a more acceptable approach.
11. Estimated PBS Usage and Financial Implications
The re-submission accepted the DUSC advice and changed its approach for estimating usage compared with the previous submission in that it appropriately based its estimates on prevalence rather than incidence. The re-submission did not present the number of patients treated rather it presented the number of episodes treated, as a patient can have more than one episode and therefore the use of episodes was considered more appropriate by the re-submission. The re-submission estimated that a total of less than 10, 000 acute severe ulcerative colitis episodes will be treated with infliximab in the first five years of listing, based on a prevalence rate of 0.16%.
The PBAC noted that the sponsor accepted the revised prevalence rate of 0.17% (in line with Access Economics report estimates) in its Pre-Sub-Committee Response (PSCR), which produced a revised number (higher) of episodes treated in the first five years of listing.
The PBAC noted that the estimated net cost to the PBS over 5 years from an infliximab listing is less than $10 million.
The re-submission claimed net cost-savings to the health budget of $10-30 million over the first five years of listing from infliximab due to reduced costs from colectomies avoided.
12. Recommendation and Reasons
The PBAC recommended extending the listing of infliximab on the PBS under the Section 100 Highly Specialised Drugs Program to include treatment of acute severe ulcerative colitis not responding to IV corticosteroids in a patient aged 6 years or greater, on a cost-minimisation basis compared with cyclosporin. The equi-effective doses estimated are infliximab: 3 infusions at days 0, 14 and 42 of 5 mg/kg and cyclosporin: 7 days intravenously 2 mg/kg followed by 90 days of oral 4 mg/kg.
The PBAC did not accept the submission’s claim of superior comparative efficacy and different and superior comparative safety of infliximab against cyclosporin. The PBAC agreed that based on the Laharie 2012 trial, infliximab is non-inferior to cyclosporin in terms of comparative effectiveness and comparative safety.
The PBAC noted the advice from Gastroenterological Society of Australia (GESA) in relation to the use of three infusions as induction therapy for acute severe ulcerative colitis, and accepted that a treatment course consisting of three doses was appropriate.
The PBAC considered that the applicability of the Jarnerot (2005) trial to the Australian population was doubtful, due to the limited data available and therefore the magnitude of the efficacy against BSC was unclear. The PBAC also considered that the clinical data from the Jarnerot (2005) trial had a high risk of bias creating uncertainties in the economic model (eg. colectomies avoided and estimated cost).
The PBAC agreed that the incremental length of stay of 6 days or 7 days may be a more reasonable assumption of hospital days saved than the re-submission’s proposed 9 days, based on Lowenberg 2013 (Lowenberg M, Duijvis NW & Ponsioen C (2013) Reduced length of hospitalisation and treatment cost with infliximab versus ciclosporin in patients with severe ulcerative colitis. American Gastroenterology Association Conference, Chicago (Abstract)). The PBAC noted that the incremental length of stay as well as cyclosporin monitoring costs would be the basis for the cost-minimised price of infliximab against cyclosporin. The PBAC has asked the Department to work with the sponsor in determining the appropriate price.
The PBAC noted the consumer comments received in relation to the submission from individuals and health care professionals highlighting the benefits from infliximab in improving the quality of life, lower indirect costs to community and avoidance of surgery. The PBAC also noted the advice received from the GESA emphasising the need for this treatment based on evidence (current treatment algorithms recognised by GESA and other international bodies). GESA also advised that due to funding constraints there was inequity in accessing infliximab from various hospitals in Australia.
The PBAC considered that infliximab remains unsuitable for inclusion in the list of PBS medicines for prescribing by nurse practitioners.
In accordance with subsection 101 3BA of the National Health Act 1953, the PBAC advised the Minister that on the basis of the material available to its November 2013 meeting, infliximab concentrate for injection should not be treated as interchangeable on an individual patient basis with any other drug or medicinal preparation.
Outcome:
Recommended
|
Name, Restriction, Manner of administration and form |
Max. Qty |
No. of Rpts |
Proprietary Name and Manufacturer |
||
|---|---|---|---|---|---|
|
INFLIXIMAB Infliximab, 100mg injection, 1 x 100 mg vial |
5a 1b |
1 1 |
Remicade |
JC |
|
|
a Public hospitals b Private hospitals
|
|||||
|
Episodicity: |
Acute |
||||
|
Severity: |
Severe |
||||
|
Condition: |
Ulcerative Colitis |
||||
|
Restriction:
|
Section 100 Highly Specialised Drugs Program Private Hospital Authority Required Public Hospital Authority Required (STREAMLINED) |
||||
|
Clinical criteria:
|
Patient must have received an infusion of infliximab for the treatment of this condition as a hospital inpatient no more than two weeks prior to the date of the authority application.
AND
Patient must be an adult aged 18 years or older, and prior to initiation of infliximab treatment in hospital have been experiencing more than six or more bloody stools per day, plus at least one of the following: (i) Temperature greater than 37.58°C; (ii) Pulse rate greater than 90 beats per minute; (iii) Haemoglobin less than 105 g/L; (iv) Erythrocyte sedimentation rate greater than 30 mm/h; OR
Patient must be a child aged 6 to 17 years inclusive, and prior to initiation of infliximab treatment in hospital have had a Paediatric Ulcerative Colitis Activity Index (PUCAI) greater than or equal to 65, with the diagnosis confirmed by a gastroenterologist, or a consultant physician as specified below.
AND
Patient must have failed to achieve an adequate response to at least 72 hours treatment with intravenous corticosteroids prior to initiation of infliximab treatment in hospital.
|
||||
|
Population criteria:
|
Patient must be aged 6 years or older
|
||||
|
Treatment criteria:
|
Must be treated by a gastroenterologist; OR Must be treated by a consultant physician [internal medicine specialising in gastroenterology, or general medicine specialising in gastroenterology].
|
||||
|
Prescriber Instructions
|
For adults aged 18 years or older, failure to achieve an adequate response to intravenous corticosteroid treatment is defined by the Oxford criteria where:
For children aged 6 to 17 years, failure to achieve an adequate response to intravenous corticosteroids means a PUCAI score greater than 45 at 72 hours.
|
||||
|
Prescriber Instructions
|
At the time of authority application, prescribers should request the appropriate number of vials, based on the weight of the patient, to provide sufficient for a single infusion at a dose of 5 mg per kg.
Before administering infliximab to a child aged 6 to 17 years, the treating clinician must have consulted with a paediatric gastroenterologist or with an institution experienced in performance of paediatric colectomy. The name of the specialist or institution must be included in the patient’s medical records.
|
||||
|
Prescriber Instructions |
Evidence that the patient meets the PBS restriction criteria must be recorded in the patient’s medical records.
|
||||
|
Administrative Advice
|
Note:
|
||||
Subsequent to ratification of the Minutes, the Committee accepted the sponsor’s request that the restriction be amended consistent with Croft 2012 and an additional publication cited in the PSCR (Lynch et al 2013). The Committee noted that the clinical criteria in this patient group is most applicable to the PBS population.
The PBAC considered that it was appropriate to amend the restriction recommended at the November 2013 PBAC meeting relating to the clinical criteria of number of stools per day (from more than six to six or more) and temperature (from 37.5°C to 37.8°C). This amendment is shown above in strikethrough and italics.
13. Context for Decision
The PBAC helps decide whether and, if so, how medicines should be subsidised in Australia. It considers submissions in this context. A PBAC decision not to recommend listing or not to recommend changing a listing does not represent a final PBAC view about the merits of the medicine. A company can resubmit to the PBAC or seek independent review of the PBAC decision.
14. Sponsor’s Comment
Janssen notes that additional data comparing infliximab to cyclosporin in a real-world setting were presented in the Pre-Sub-Committee Response from the 2008 and 2010 UK IBD audit (Lynch et al 2013; APT: 38, 935-945).
Janssen thanks the PBAC for recommending Remicade (infliximab) for the treatment of acute severe ulcerative colitis. This will provide equity of access to infliximab in Australia for the treatment of acute severe ulcerative colitis.




