Levonorgestrel, intrauterine system, 13.5 mg, Jaydess® - March 2014
PDF printable version of this page
Public Summary Documents
Product: LEVONORGESTREL, intrauterine system, 13.5 mg, Jaydess®
Sponsor: Bayer Australia Limited
Date of PBAC Consideration: July 2013
The July 2013 levonorgestrel Public Summary Document is reproduced below with the addition of an ‘ADDENDUM’ at the end of the document to convey the March 2014 PBAC consideration.
1. Purpose of Application
The submission sought a Restricted benefit listing for contraception for up to three years.
2. Background
Intrauterine levonorgestrel contraceptive system (releasing approximately 12 micrograms per 24 hours) (LCS12) had not previously been considered by the PBAC.
3. Registration Status
The PBAC noted that both the clinical evaluation report and the TGA Delegates overview were available at the time of the consideration by the PBAC in July 2013.
LCS12 was registered by the TGA on 18 September 2013 for the following indication:
- Contraception for up to 3 years
4. Listing Requested and PBAC’s View
Restricted benefit
Contraception for up to 3 years
The PBAC noted and agreed with the Secretariat suggested amendment to remove the duration of contraception
5. Clinical Place for the Proposed Therapy
The submission stated that more than 70% of Australian women currently use contraception. However, unplanned pregnancy occurs in up to 50% of women which can be both a clinical and economic burden.
The clinical management algorithm is based on the currently available contraception options listed on the PBS and concurs with the management algorithm of the main comparator, Mirena. The intended use of LCS12 is for women who require progesterone only, long acting reversible contraception (LARC).
6. Comparator
The submission nominated Mirena and Implanon as the main comparators. Mirena is a levonorgestrel releasing intrauterine system but has a larger frame (32 x 32 mm), compared to LCS12 (28 x 28 mm) and releases more levonorgestrel per 24 hours (20 µg/day) than LCS12. Implanon is a single rod sub-dermal implant containing 68 mg of etonogestrel, which is a pharmacological analogue of levonorgestrel. The release rate for Implanon is 35-40 µg/day.
The PBAC considered that Mirena is the most appropriate comparator due to the similarity in devices. The populations using injection or sub-dermal implant are likely to differ in terms of their preferences, from those using an intra-uterine contraceptive device. In addition, given that Mirena is already available on the PBS, the PBAC agreed that any substitution from Implanon to an intrauterine system has likely already occurred.
7. Clinical Trials
In comparing LCS12 to Mirena, the submission presented one head-to-head randomised phase II trial (LCS12 Phase 2 study) and one supplementary randomised phase III clinical trial (Phase 3 Study; Protocol 310442-91665) comparing LCS12 and LCS16.
In comparing LCS12 to Implanon, the submission presented an indirect comparison of one RCT comparing LCS12 with Mirena, one RCT comparing Mirena with Norplant/Norplant-2 (another sub-dermal progestogen releasing contraceptive) and eight randomised controlled trials comparing Norplant/Norplant-2 with Implanon. Three meta-analyses were also included in the submission, comparing Mirena to other forms of contraception
None of these studies had been published at the time of submission.
8. Results of Trials
The results of primary (number of pregnancies) and secondary outcomes (bleeding patterns) were presented. For the comparison with Mirena, the PBAC noted that non-inferiority was largely inferred by overlapping confidence intervals and that the number of pregnancies was very low using either method of contraception. However, the PBAC further noted the TGA Delegate’s observation that the observed efficacy of LCS12 in the registration studies was consistent with the adopted guidelines (EMA CHMP guidelines for Pearl Index criteria) and comparable but nevertheless lower than Mirena, which may be an issue at individual user level.
The PBAC considered that the data to support the comparison with Implanon were difficult to interpret but overall the event rates showed that both products are effective methods for contraception.
While the submission provided a comparison of bleeding outcomes associated with use of each product, these studies were difficult to interpret in the Australian setting, as acceptability of bleeding is influenced by cultural considerations and there may have been considerable reporting bias. There was little data to support any meaningful differences between products.
With regard to comparative harms, the PBAC noted that no formal indirect comparisons were done for the safety outcomes for either Mirena or Implanon. The clinical claim of non-inferiority of comparative safety was based on comparing descriptive analyses of adverse events.
9. Clinical Claim
The submission described LCS12 as non-inferior in terms of comparative effectiveness and non-inferior in terms of comparative safety over Mirena.
The submission further described LCS12 as non-inferior in terms of comparative effectiveness and non-inferior in terms of comparative safety over Implanon.
The PBAC was yet to accept these claims, as the PBAC noted the following:
- Numerically higher rates of unintended pregnancy for LCS12 compared to Mirena
- The LCS Phase 2 Study was not powered to test inferiority and an absence of any formal statistical analysis; the reliance on a two step indirect comparison of LC12 to Implanon in terms of bleeding outcomes, amenorrhoea, frequent bleeding and prolonged bleeding. There are exchangeability issues between the different trials including different inclusion and exclusion criteria, and the disparate healthcare systems (e.g. Indonesia versus Australia) and regions where the trials were conducted.
- The TGA Delegate’s observations of comparable but lower efficacy of LCS12 compared to Mirena, and, higher relative risk of ectopic pregnancy with LCS12 compared to Mirena; and
- The absence of a recommendation by the TGA Delegate
10. Economic Analysis
A cost-minimisation analysis was presented based on cost-equivalence of LCS12 with Mirena and Implanon, including all health care costs to both the PBS and the MBS. The total costs included the cost of the implant, health care costs and insertion and removal costs. The total costs and the average annual cost for each implant was estimated, with the average annual cost for each implant based on the in-situ times presented in the submission.
The PBAC noted an error in the cost analysis arising from the submission’s use of Mirena’s published price which incorporated a weighting for another subsidised indication (menorrhagia). The cost-minimisation analysis was redone during the evaluation.
Based on a weighting of Mirena and Implanon derived from market research, the submission’s proposed price was estimated and a revised price was calculated during the evaluation for the contraception indication only. The proportion of patients on Mirena or Implanon from the submission’s market research was converted to units replaced on the PBS according to the differing in-situ times for the products.
The PBAC noted that the cost-minimisation analysis was most sensitive to the substitution weightings, the basis of which were not accepted by the PBAC at this stage because the PBAC considered that the market research was poorly substantiated. The PBAC noted that the greater the weighting that is placed on Mirena, the lower the proposed cost of the LCS12 would need to be to maintain cost-minimisation
11. Estimated PBS Usage and Financial Implications
The PBAC considered that the substitution for Implanon was overestimated and more substitution would be from Mirena.
The submission’s estimated total net cost to the PBS was between $100,000-$200,000 over the first 5 years, with estimated cost savings in the first 2 years of listing and again in year 5. With the revised price for LCS12, this net cost to the PBS over the first 5 years was revised to be greater than $200,000. The financial implications are yet to be verified.
Overall, the total cost per year to the Government was estimated in the submission to be less than $10 million over the first five years of listing.
The PBAC considered that the assumptions used to determine the financial costs were likely to overestimate reductions in cost. The PBAC noted that if Mirena is the only comparator and substituted at 100%, overall, over the 5 years, the total cost to Government, was estimated by a sensitivity analysis conducted during the evaluation in the range of $10 million to $30 million.
12. Recommendation and Reasons
The PBAC deferred the submission in the absence of a positive TGA Delegate’s recommendation to register LCS12, at the time of consideration by the PBAC. The PBAC noted the concern in the TGA Delegates overview that while the ectopic pregnancy rate for LCS12 is low, lack of comparison with Mirena in the pivotal study raises concern that there may be an undetected clinically significant difference in risk of pregnancy, and specifically ectopic pregnancy.
The PBAC indicated that it would reconsider the submission as it currently stands once a positive Advisory Committee on Prescription Medicines (ACPM) outcome is received. The PBAC did not request that the Sponsor provide any additional information prior to reconsideration, apart from updating the status of the TGA application
13. Context for Decision
The PBAC helps decide whether and, if so, how medicines should be subsidised in Australia. It considers submissions in this context. A PBAC decision not to recommend listing or not to recommend changing a listing does not represent a final PBAC view about the merits of the medicine. A company can resubmit to the PBAC or seek independent review of the PBAC decision.
14. Sponsor’s Comment
Bayer does not agree with Committee’s current view regarding comparator weighting nor the stated financial impact estimates and will bring forward a further submission addressing these points. In addition, Bayer will also bring forward the positive ACPM outcome within the re-submission. Bayer is committed to continue working with the PBAC to facilitate access of an additional long acting reversible contraceptive (LARC) option for Australian women.
ADDENDUM
Public Summary Document
Product: LEVONORGESTREL, intrauterine system, 13.5 mg, Jaydess®
Sponsor: Bayer Australia Limited
Date of PBAC Consideration: March 2014
1. Purpose of Application
The minor resubmission sought to provide the PBAC with an update on the TGA registration status following the PBAC’s July 2013 deferral of a major submission seeking listing of levonorgestrel intrauterine system releasing approximately 12 micrograms per 24 hours (hereon referred to as Jaydess) on the PBS as a Restricted Benefit, for use in contraception.
The minor resubmission provided further information in addition to the update on the TGA registration status, to address various concerns raised during the July 2013 evaluation process predominantly concerning what the appropriate comparator is for the economic evaluation.
2. Background
At the July 2013 meeting, the PBAC deferred a major submission seeking a Restricted benefit listing for contraception for up to 3 years in the absence of a positive TGA Delegate’s recommendation (the TGA Delegate had not proposed an action), at the time of consideration by the PBAC. The PBAC noted the concern in the TGA Delegate’s Overview that while the ectopic pregnancy rate for Jaydess is low, a lack of comparison with Mirena in the pivotal study raises concern that there may be an undetected clinically significant difference in risk of pregnancy, and specifically ectopic pregnancy.
The PBAC indicated that it would reconsider the submission, once a positive ACPM outcome is received. The PBAC did not request that the sponsor provide any additional information prior to reconsideration, apart from updating the status of the TGA application.
3. Requested Listing and PBAC’s View
The requested listing was unchanged from that requested in July 2013.
Listing was sought on a cost-minimisation basis to a mixed comparator of levonorgestrel 52 mg in an intrauterine drug delivery system (hereon referred to as Mirena) and etonogestrel 68 mg sub-dermal implant (hereon referred to as Implanon NXT).
The PBAC recalled that it had agreed in July 2013 with the Secretariat suggested amendment to remove the duration (up to 3years) of contraception from the restriction.
The PBAC noted the potential for leakage outside the proposed PBS restriction to women experiencing idiopathic menorrhagia and for the prevention of endometrial hyperplasia during oestrogen replacement therapy but did not consider this leakage likely to be a significant issue.
4. Clinical Place for the Proposed Therapy
The July 2013 submission’s depiction of the place of Jaydess in the clinical treatment algorithm placed Jaydess as a direct alternative to other long acting, reversible contraception. This is shown below.
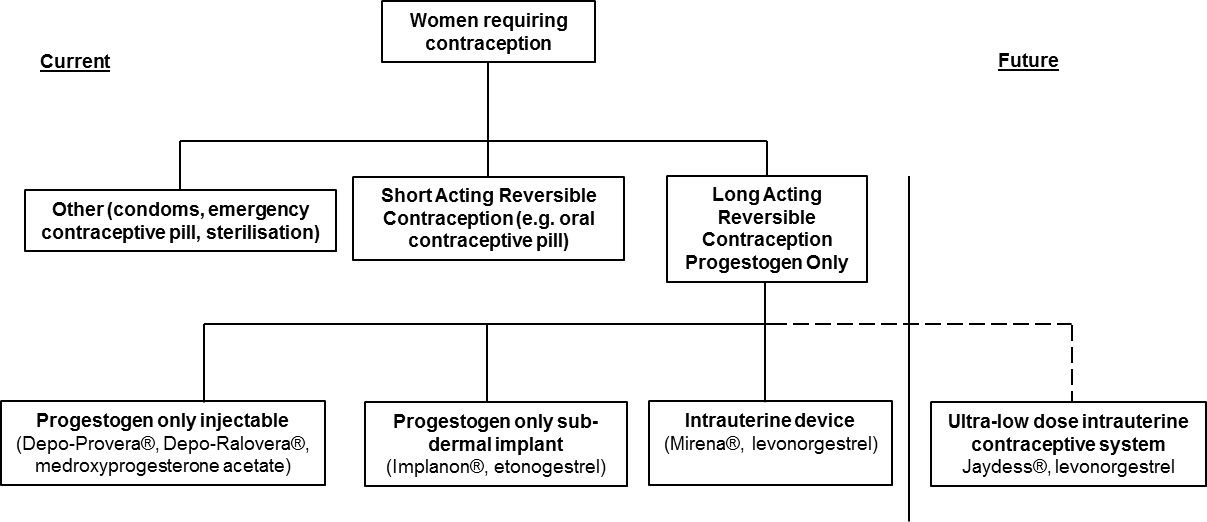
The minor re-submission placed heavy emphasis on arguing that Jaydess would substitute in practice for Implanon in addition to Mirena, particularly in young, nulliparous (yet to give birth) women due to Jaydess’ smaller, physical design size compared to Mirena. The re-submission’s additional reasons to support this view included the following claims:
A claimed alignment with current practice consensus to increase uptake of long-acting reversible contraception particularly in young, nulliparous women;
- Increased patient acceptance and advantage in nulliparous women due to the smaller design size.
- The Product Information of Mirena states that “Mirena is not the method of first choice for young nulligravid women”.
- Claimed physician misconceptions about the suitability of intra-uterine contraceptive devices in nulliparous women.
The PBAC noted that Jaydess is an intrauterine device that delivers the hormone levonorgestrel and was of the opinion that Jaydess would provide an alternative levonorgestrel contraceptive option for women requiring contraception delivered via an intrauterine device.
5. Comparator
The nominated comparators of Mirena and Implanon remained unchanged from July 2013.
The PBAC recalled that in July 2013, it had considered that Mirena is the most appropriate comparator due to the similarity in devices. This view remained unchanged as the PBAC still considered that the patient populations using injection or sub-dermal implant are likely to differ in terms of their preferences, from those using an intra-uterine contraceptive device. Despite the minor re-submission’s various arguments outlined above, given that Jaydess contains the same pharmaceutical drug as Mirena and both have the same manner of administration (i.e. intrauterine) and in the context of the proposed broad restriction, the PBAC considered that for a majority of patients, Jaydess would substitute for Mirena.
The PBAC considered that if Implanon was to be accepted as a main comparator, the submission would have to adequately define the patient population for which Implanon is the main comparator. The PBAC noted that the submission had attempted to identify this patient population to be young, nulliparous women but that the requested restriction did not strictly limit PBS subsidy to such patients and that defining such a patient population, as distinct from identifying, had not been attempted in the submission.
6. Clinical Trials
The clinical trial evidence remained unchanged from the July 2013 submission.
7. Results of Trials
The results of the primary (number of pregnancies) and secondary outcomes (bleeding patterns) remained unchanged from the July 2013 submission.
With respect to the comparison of Jaydess to Mirena, the PBAC recalled that non-inferiority was largely inferred by overlapping confidence intervals and that the number of pregnancies was very low using either method of contraception. The PBAC further recalled the TGA Delegate’s observation that the observed efficacy of Jaydess in the registration studies was consistent with the adopted guidelines (EMA CHMP guidelines for Pearl Index criteria) and comparable but nevertheless lower than Mirena which may have been an issue at individual user level.
With respect to the comparison of Jaydess to Implanon, the PBAC recalled that the data to support this comparison were difficult to interpret but overall the event rates showed that both products are effective methods for contraception.
The PBAC recalled that while the July 2013 submission provided a comparison of bleeding outcomes associated with use of each product, these studies were difficult to interpret in the Australian setting, as acceptability of bleeding is influenced by cultural considerations and there may have been considerable reporting bias. There was little data to support any meaningful differences between products.
Results for comparative harms remained unchanged from the July 2013 submission.
The PBAC recalled that no formal indirect comparisons were done in the July 2013 submission for the safety outcomes for either Mirena or Implanon. The clinical claim of non-inferiority of comparative safety was based on comparing descriptive analyses of adverse events.
The PBAC further recalled in July 2013 that the TGA Delegate’s observation that the registration data indicated a higher relative risk of ectopic pregnancy (1.8 fold) with Jaydess compared to Mirena and that this was a concern. However, the PBAC felt reassured of this safety concern after considering the ACPM’s August 2013 meeting minutes regarding this issue.
8. Clinical Claim
No new clinical claims were made in the minor re-submission.
The PBAC recalled that the July 2013 submission described Jaydess as non-inferior in terms of comparative effectiveness and non-inferior in terms of comparative safety over Mirena.
The PBAC further recalled that the July 2013 submission described Jaydess as non-inferior in terms of comparative effectiveness and non-inferior in terms of comparative safety over Implanon.
The PBAC considered that the outcomes of the August 2013 ACPM meeting and finalisation of Jaydess’ TGA registration were now sufficient to enable it to accept these clinical claims.
9. Economic Analysis
The economic evaluation remained unchanged from the July 2013 submission.
The PBAC recalled that the July 2013 submission presented a cost-minimisation analysis based on cost-equivalence of Jaydess with Mirena and Implanon, including all health care costs to both the PBS and the MBS. The total costs included the cost of the implant, healthcare costs and insertion and removal costs. The average annual cost for each implant was estimated based on the in-situ times presented in the submission (2.52 years for Jaydess, 3.62 years for Mirena, and, 2.17 for Implanon NXT).
The PBAC recalled that there was an error in the cost analysis arising from the submission’s use of Mirena’s published price which incorporated a weighting for another subsidised indication (menorrhagia). The correct price was identified during the evaluation for the cost minimisation calculation for Mirena for contraception. The cost-minimisation analysis was redone during the July 2013 evaluation.
The PBAC did not accept the proposed substitution weightings (51% for Mirena, 49% for Implanon NXT) which remained unchanged from the July 2013 submission. Despite the resubmission’s numerous arguments revolving around Jaydess’ smaller physical size, references to Mirena’s product information, consensus statements and perceived prescriber misconceptions that aimed to support the use of Implanon NXT as a comparator, the PBAC noted that both Jaydess and Mirena contain levonorgestrel as the active drug and that both deliver levonorgestrel via an intrauterine delivery device and so remained unconvinced that Jaydess would substitute for Implanon NXT in up to 49% of patients requiring long acting, reversible contraception excluding depot injections. Therefore, the PBAC considered the economic comparison should have been limited to comparing Jaydess to Mirena.
In summary, the PBAC considered that Jaydess would be acceptable for PBS listing if Jaydess were cost minimised against Mirena in such a way that the annual drug/device treatment costs to Government (including costs to the MBS) were equivalent between the two products. The PBAC considered that the pricing of Jaydess would therefore need to factor in the shorter duration of action compared to Mirena (the approved Product Information for Jaydess currently states 3 years duration whilst for Mirena, it is 5 years duration), and, the resulting increased MBS item costs related to related to removal/insertion of Jaydess devices arising from its shorter duration of contraceptive cover compared to Mirena.
10. Estimated PBS Usage and Financial Implications
Estimated PBS usage and financial implications remained unchanged from the July 2013 submission, as did the PBAC’s views.
The PBAC noted the minor resubmission’s claim that the July 2013 evaluation’s sensitivity analysis of the total cost to government over 5 years of listing assuming 100% substitution for Mirena, is incorrect and that there should be an amendment of the Minutes. The PBAC disagreed with this claim, noting that the results of the sensitivity analysis were plausible based on the following assumptions:
The proposed prices of Jaydess and Mirena as proposed in the submission resulted in a small annual incremental cost difference against Jaydess taking into account the different in-situ timeframes;
- Consistent with the approved Product Information, Mirena is left in-situ for 5 years whilst Jaydess is left in-situ for 3 years, meaning that patients swapped from Mirena to Jaydess in years 1 and 2 of listing would have to return to the prescriber in years 4 and 5 respectively to have another device prescribed (increasing PBS drug costs) and inserted (increasing MBS costs);
- MBS device insertion/removal costs are $53.55 per event; and
- At least 60,000 Mirena prescriptions are substituted for, given the 100% substitution assumption, in each of the 5 years of listing and which is based on 100,908 Mirena prescriptions (PBS item code 8633J) being processed by Medicare Australia in the 2012/13 financial year for which 59.2% are assumed to be for contraception.
The PBAC noted the potential for increased MBS costs associated with increased insertion/removal costs driven by Jaydess’ shorter duration of contraceptive cover (3 years versus 5 years for Mirena).
11. PBAC Outcome
The PBAC recommended listing Jaydess as a Restricted benefit for contraception.
The PBAC did not recommend that the Safety Net 20 Day rule be applied.
The PBAC recommended that Jaydess is suitable for inclusion in the PBS medicines for prescribing by nurse practitioners within collaborative arrangements, consistent with the currently PBS listed intrauterine contraceptive device Mirena. The PBAC noted that Implanon NXT is also available for PBS prescribing by midwives but did not recommend Jaydess for prescribing by midwives for consistency with Mirena.
Recommendation:
Add new item:
|
Name, Restriction, Manner of administration and form |
Max. Qty |
No. of Rpts |
Proprietary Name |
Manufacturer | |
|---|---|---|---|---|---|
|
LEVONORGESTREL
levonorgestrel 13.5 mg drug delivery system: intrauterine, 1 system |
1 |
0 |
Jaydess |
BN |
|
|
Indication: |
Contraception |
|||||
|
Restriction: |
Restricted benefit |
|||||
12. Context for Decision
The PBAC helps decide whether and, if so, how medicines should be subsidised in Australia. It considers submissions in this context. A PBAC decision not to recommend listing or not to recommend changing a listing does not represent a final PBAC view about the merits of the medicine. A company can resubmit to the PBAC or seek independent review of the PBAC decision.
13. Sponsor’s Comment – March 2014
Bayer welcomes the PBAC’s acceptance of the clinical claims of non-inferiority to both Implanon and Mirena. Bayer also acknowledges PBAC advice that if Implanon were to be accepted as a main comparator, the sponsor would have to adequately define the patient population for which Implanon is the main comparator and strictly limit PBS subsidy to such patients. Bayer will bring forward robust utilisation evidence in a re-submission clearly defining both Implanon and Mirena patient population by age groups, with a proposal meeting the PBACs requirements for Implanon inclusion as a relevant economic comparator.




