FAQ – Responsible Persons
Please note: The information on this page is no longer current and is only included here for historical reference.
Current information is maintained on the Price Disclosure (SPD) page.
- How do I report Incentives data?
- How are over-the-counter (OTC) products treated under price disclosure?
- How do I calculate a new Brand Premium / Special Patient Contribution after a Price Disclosure Determination is made?
How do I report Incentives data?
Incentives can be reported separately with each data submission, or included by reporting net sales which already account for any incentives (but not both).
Regardless of how incentives are reported, the methodology for recording and allocation of incentives must be well documented and consistent with Responsible Persons' financial accounting policies.
Also note that any incentives already incorporated in the data reported for sales revenue should not be disclosed separately as incentive data (to prevent double counting of incentives).
Please refer to the Collection section of the Procedural and Operational Guidelines for more information about incentives.
How are over-the-counter (OTC) products treated under price disclosure?
The Responsible Person for a PBS listed brand* subject to price disclosure must report on all sales (PBS, over the counter, private script etc) for any items that share drug, form/strength, manner of administration and brand name with the PBS listed brand. This may include different pack sizes which do not have approved prices on the PBS.
Differently named brands specifically intended for over the counter sales, but which share drug, form/strength, manner of administration as a PBS listed brand which is subject to price disclosure, should not be reported. Sales of a particular item should be reported unless it is clear that they meet this criteria i.e. if a particular item could be used to fill a PBS script, then it should be reported.
For example, assume the PBS listed pharmaceutical item "Sample drug, Tablet 100 mg, oral" is subject to price disclosure, but "Sample drug, Tablet 50 mg, oral" does not have an approved price on the PBS. The Responsible Person for the PBS listed brand "Samplex" for the 100 mg tablet must report on the following:
| Drug | Form/Strength | Pack size | MOA | Brand | Reporting required |
|---|---|---|---|---|---|
| Sample drug | Tablet 100 mg | 100 | Oral | Samplex | Yes |
| Sample drug | Tablet 100 mg | 50 | Oral | Samplex | Yes |
| Sample drug | Tablet 100 mg | 24 | Oral | Samplex Rapid (OTC) | No |
| Sample drug | Tablet 100 mg | 48 | Oral | Samplex Rapid (OTC) | No |
| Sample drug | Tablet 50 mg | 100 | Oral | Samplex Rapid | No |
*a brand of pharmaceutical item as determined under section 85 of the National Health Act 1953. 'Pharmaceutical item' is the declared drug in the form and with the manner of administration determined under s85.
How do I calculate a new Brand Premium / Special Patient Contribution after a Price Disclosure Determination is made?
A Brand Premium / Special Patient Contribution for a brand of a pharmaceutical item that has a determination in force, under subsection 85B(3) of the National Health Act (1953) (the Act), can be calculated by following the below steps:
Step 1: Identify the prices to be used in the calculation
- Approved (Commonwealth) Price to Pharmacists (AAP) before the applicable Reduction Date
- Adjusted Approved (Commonwealth) Price to Pharmacists (AAAP) listed on the Price Disclosure Determination
- Current Claimed Price (current Manufacturer’s Price to Pharmacists) before the applicable Reduction Day
Step 2: Calculate the new Claimed Price (New Manufacturer’s Price to Pharmacists)
To determine a new Claimed price, calculate the reduction percentage that needs to apply to the current Claimed Price, as prescribed in subsection 99ADH (4) of the Act. This method is tabled below:
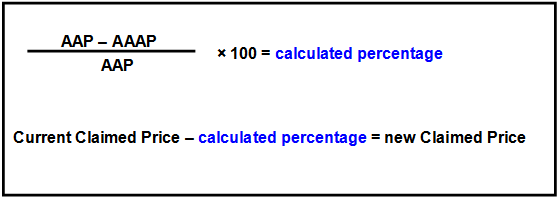
Step 3: Calculate the Brand Premium / Special Patient Contribution
Calculate the Manufacturer’s Dispensed Price for Maximum Quantity (MDPMQ) (incl. Brand
Premium)
New Claimed Price + applicable Pharmacy Mark Up + applicable Dispensing Fee = MDPMQ
Calculate the Dispensed Price for Maximum Quantity (DPMQ) (no Brand Premium)
AAAP + applicable Pharmacy Mark Up + applicable Dispensing Fee = DPMQ
Calculate the Brand Premium / Special Patient Contribution
MDPMQ – DPMQ = new Brand Premium
Example:
1) Identify the prices required for the calculation
a. AAP = $10.00
b. AAAP = $8.50
c. Current Claimed Price = $12.00
2) Calculate the new Claimed Price
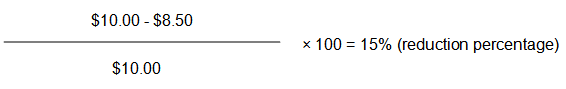
$12.00 (current Claimed Price) - 15% = $10.20
The new Claimed Price for this brand of pharmaceutical item is $10.20
3) Calculate the new Brand Premium / Special Patient Contribution
Calculate MDPMQ
$10.20 + ($10.20 × 15%) + $6.42 = $18.15
(new Claimed Price + applicable Pharmacy Mark Up + applicable Dispensing Fee = MDPMQ)
Calculate DPMQ
$8.50 + ($8.50 × 15%) + $6.42 = $16.20
(AAAP + applicable Pharmacy Mark Up + applicable Dispensing Fee = DPMQ)
Calculate Brand Premium / Special Patient Contribution
$18.15 - $16.20 = $1.95
(MDPMQ – DPMQ = Brand Premium)




