AMWG Interim Report to Government - Attachment A
The Effects of Statutory Price Reductions on the listing of New Medicines
Executive Summary
The Department of Health and Ageing (DoHA) and Medicines Australia (MA) through the Access to Medicines Working Group (AMWG), have examined the possible impacts on the listing process for mandatory price reductions from 1 August 2008 for medicines on the new F2 formulary. Both parties agree that, in principle, a subset of new medicines seeking listing on the Pharmaceutical Benefits Scheme (PBS) may be impacted in the future as a result of PBS reform. In AMWG discussions, DoHA argued the issue will be relatively contained and that the current system of evaluating medicines for listing on the PBS is sufficiently flexible to handle any issues that may arise, while MA suggested that it will be more widespread and argued that changes are needed. Both parties have discussed several options, involving substituting an F1 comparator or F1 price for evaluations where an F2 product is the comparator.
Introduction
This paper presents a discussion by the Access to Medicines Working Group (AMWG) of possible effects of statutory price reductions for Pharmaceutical Benefits Scheme (PBS) Formulary 2 (F2) medicines on the cost effectiveness assessment of new Formulary 1 (F1) medicines seeking PBS listing.
Background
The AMWG, consisting of Department of Health and Ageing (DoHA) and Medicines Australia
(MA) members, was established to consider issues relating to timely and appropriate
access to effective new medicines on the PBS, and issues resulting from the 2006 PBS
reforms.
The AMWG was, amongst other things, tasked with considering “the possible impacts
on the listing process for mandatory price reductions from 1 August 2008 for medicines
on the new F2 formulary” (The Australian Government, Department of Health and Ageing
and Medicines Australia. Access to Medicines Working Group, Communiqué July 2007.
This paper builds on discussions and analyses on this issue undertaken by MA and DoHA
from mid-2006 through to 2008.
Context
PBS Reform
In November 2006, the then Government announced a number of changes to the PBS to
protect patients from higher out of pocket costs, get better value from market competition
among brands of generic (off-patent) medicines and recognise the importance of world-class
life-enhancing drugs to patients. The main change is in the way in which the Government
prices medicines that are operating in a competitive market (F2). These medicines
will take a series of price drops, and eventually will move to a system where the
price they are sold to pharmacists will be the same price paid by the Government.
In its response to Medicines Australia’s 2007 election document prior to last year’s
federal election, the then Federal Opposition signalled what would become the new
Government’s support for PBS reform and commitment to ensuring patients have timely
access to new medicines:
“A Rudd Labor Government will implement the program of PBS reform legislated in June
2007. However, in doing so, we will continue to monitor both the effectiveness of
the reforms and their impact on consumers, the generic medicines sector and other
stakeholders … Federal Labor is committed to ensuring timely access to PBS medicines
for the Australian public (Federal Labor Response to Medicines Australia Election
Statement Medicines Matter to Australians, Office of Nicola Roxon MP, Shadow Minister
for Health, Canberra, 22 November 2007)."
The Basis for PBS listing
Proposals for listing new medicines or medicinal products on the PBS, or making changes
to existing listings, must be considered by the Pharmaceutical Benefits Advisory Committee
(PBAC), which is required to take into account the effectiveness and cost of a medicine
compared with other drug or non-drug therapies when reaching a decision. The National
Health Act 1953 Section 101(4) stipulates that a positive recommendation by PBAC is
required before any new medicine can be listed.
The economic basis of a PBAC recommendation usually follows one of two approaches:
- A cost-effectiveness analysis allows a proposed new drug to be listed with a price advantage over its comparator, provided the extent of this price advantage can be justified by improved health outcomes, efficacy or safety.
- A sub-set of cost-effectiveness analysis, a cost-minimisation approach applies where there are insufficient gains in health outcomes to justify a higher price for the proposed drug over currently listed alternatives.
The statutory price cuts resulting from PBS Reform may impact on the listing of new
medicines where the listing has been on the basis of either cost-effectiveness or
cost-minimisation analysis.
Assessment of potential impact
What is the issue?
As a result of PBS reform, some new innovative medicines will be compared to F2 medicines for pricing comparator purposes. This is likely to lead to some new medicines being offered a lower listing price in F1. On the basis of consultations with its membership, MA believes that there is a possibility that Australians’ future access to some new innovative medicines may be delayed or compromised as a result of PBS Reform.
As the economic evaluation of a new medicine is assessed by comparison with an existing treatment, it follows that from time to time a new medicine will be compared with an existing F2 medicine. Normally, if the claim is that it is “no worse than” an existing PBS-listed medicine (ie cost-minimised), it would be expected to match the total cost of treatment using the existing medicine. If the claim is that the new medicine is better (ie cost-effective), then it would be expected that extra expenditure through the PBS would reflect the value of that measurable health improvement.
The issue arises because F2 medicines will experience statutory price reductions from 1 August 2008, and may experience further reductions from 1 August 2009 due to the new price disclosure pricing policy. This may impact on the listing of new F1 medicines where the comparator is a F2 medicine that has experienced a price reduction due to the reforms.
Analysis (what are the facts)
MA and DoHA have undertaken retrospective analyses to measure the likely proportion of comparators which will be listed in F2 by profiling recent PBAC outcomes (refer Appendix D and Appendix E).
This analysis has identified that between 15% and 21% of new PBS applications in recent years were evaluated against medicines that are now listed in F2. Seven to eight per cent of successful applications used comparators that are now listed on the F2T formulary, where a 25% price reduction will occur on 1 August 2008. A further 8% to 14% of successful applications have a comparator in F2A where there is a possibility of significant price reductions from 1 August 2009 as a result of price disclosure. This might also affect the listing of a new drug. A greater proportion of new drugs will be compared against medicines listed on F1, a smaller proportion of which will experience price reductions under the PBS Reforms compared to the previous situation.
An examination of 113 PBAC decisions from November 2005 to November 2006 was carried out through information available in public summary documents (PSDs). The analysis indicated that for cost effectiveness submissions:
- the comparator was a F1 medicine or placebo in approximately 85% of cases; and
- the comparator was a F2 medicine in approximately 15% of cases.
As part of AMWG’s work, MA also undertook a prospective survey of its member companies to understand the extent to which new medicines seeking PBS listing in the future would have an F2 comparator. From responses from 16 MA member companies, a total of 37 new chemical entities and indications for which a company had expected to seek a PBS listing in the next five years were identified as expected to have an F2 comparator. To put this is perspective, given that there will be at least 15 PBAC meetings over this time, this means, on average between 2 and 3 submissions per PBAC meeting may have an F2 comparator. It is worth noting that the MA survey most likely underestimates the actual extent of the problem as the absolute numbers provided are derived from a small sample size of the membership (16 out of 50 member companies).
Perspectives on likely potential impact
Both DoHA and MA agree, in principle, that the evaluation of some new medicines will be against comparators that are in the F2 formulary. However, DoHA and MA have differing views on the extent to which this is likely to be an issue for the availability of new medicines in the future. The extent to which comparator price reductions will affect the listing of new medicines is dependent on a number of factors. These include: the number of new medicines that will have an F2 comparator; the ultimate success of the price disclosure policy in delivering ongoing savings in the F2 market; and the combined impact of all price reductions in establishing the relative cost-effectiveness of new medicines.
MA view
During AMWG discussions, MA suggested that this change has created a problem. This is due to the number of medicines that may have an F2 comparator in the future, the number of medicines experiencing patent expiry in the next few years, and the fact that several categories of new medicines may be adversely affected and the likelihood of larger than expected price reductions in F2 as a result of the Government’s policy of price disclosure. MA’s view is that it is preferable to pre-empt any potential unintended consequence of PBS reform and introduce sufficient flexibility in the listing process to ensure that ‘at-risk’ medicines can be dealt with on a case by case basis, particularly because PBS Reform was not intended to negatively impact on any important medicine. MA argues that the two formularies are separate, with prices in F1 determined by evidence-based medicine and cost-effectiveness analysis, while in F2 market competition determines price and that given the different markets, there should be no price linkage between F1 and F2 medicines at the time of price setting as well as price maintenance after listing.
DoHA view
In discussion at AMWG, DoHA suggested that given the apparent small numbers of PBAC submissions in the past that have had an F2T comparator, and the uncertain likelihood of further price reductions in F2, the issue is likely to be confined to a subset of medicines and that the current system of evaluating medicines for listing on the PBS is sufficiently flexible to handle issues that may arise. DoHA therefore does not agree there is a problem.
DoHA notes that many policies that affect pricing and therefore comparator pricing and cost effectiveness have been introduced over time. The price reductions applying from 1 August 2008 are inherently no different to previous pricing reductions. The fundamental application of cost-effectiveness when considering new medicines does not alter with the introduction of price reductions flowing from PBS Reform, nor will the existing flexibility of the PBAC be altered by the pricing reductions. Constraining cost effectiveness comparators to within the F1 and F2 formularies is an artificial construct, as comparators can be drawn from any formulary or from outside the PBS for the purposes of price setting. DoHA suggests that on average the size of the price reduction required to significantly impact on the cost-effectiveness equation is larger than the size of the mandatory price reductions being implemented as part of PBS reform, although this varies with different medicines. DoHA agrees that there will be some applications using a F2 comparator which will experience these impacts, and believes that perhaps in some circumstances PBAC will need to take that into consideration.
Options for managing impact of F2 comparator price reductions
The AMWG discussed a range of possible approaches for ensuring that price reductions that occur as a result of PBS reform, do not unintentionally affect the listing of new medicines on the PBS. Some of the options initially proposed by Medicines Australia in the discussions, such as CPI adjustment of F1 medicine prices and adjusting the PBAC’s incremental cost-effectiveness ratio (ICER) were deferred for later discussion under other AMWG terms of reference.
Discussion then focussed on three options, with some variations, to address the situation where a new single brand medicine is seeking listing on the PBS but its clinical comparator (the medicine most likely to be replaced in clinical practice) is an F2 medicine, and therefore may be more difficult to gain a higher price than the comparator medicine as a result of the Government’s PBS reform policy. In the discussion of each option below, a summary of the strengths and weaknesses of the options discussed during AMWG deliberations is provided. Examples of how the options might work are described in Appendix G.
All the following options will increase the cost to Government of PBS medicines more
than would be the case if new F1 medicines were evaluated against the appropriate
F2 comparator.
a) Use of F1 comparator
When a company submits a new medicine for listing on the PBS which would otherwise
be compared against an F2 medicine, the comparator against which it will be considered
will automatically be another F1 product. Where a relevant F1 product does not exist
the comparator is placebo for standard management. Note that the new medicine will
only achieve a higher price than currently listed F1 medicines if it demonstrates
superior outcomes supporting acceptable cost-effectiveness, the same as the current
situation. Thus the principle of the need to establish cost effectiveness is unchanged
by this option. A variant to this is that the company may choose whether it would
exercise its option to have an F1 comparator.
Strengths: Simple and easy to understand and achieves the quarantining of any changes in the
pricing of medicines on the F2 formulary affecting the determination of the cost effectiveness
of a new medicine. Sponsors will still need to satisfy that medicines are effective
and safe but the cost-effectiveness of the medicine will not be influenced by the
impact of changed pricing arrangements for existing generic medicines as a result
of the Government’s PBS reform policy. Can be implemented within the boundaries of
the current F1/F2 split in PBS reform.
Weaknesses: Such an approach is at odds with current practice of comparator choice and cost-effectiveness
analysis. Excluding as potential comparators all products listed in F2, regardless
of their appropriateness as defined by current clinical practice, compromises the
principles of cost-effectiveness analysis as defined by the current PBAC guidelines.
This option may also give rise to undue incentives to bring non-innovative products
to market. There may also be situations where a company would prefer to have an F2
comparator and this option would prevent them from doing so, even if that might be
most appropriate.
b) Use of F1 price for pricing purposes only
Under this option, where a company is seeking the listing of a new medicine and its
clinical comparator (the medicine most likely to be replaced in practice) is in F2,
that comparator is used for clinical evaluation, but an equivalent F1 price is substituted
in the economic evaluation. Sponsors would need to establish relative clinical effectiveness
against the relevant comparator product regardless of whether that medicine is listed
on F1 or F2. For the purposes of establishing cost-effectiveness and price setting,
however, the comparator would be adjusted to reflect equivalent F1 pricing, either
by reference to an equivalent F1 comparator or re-calibrating the F2 comparator’s
price to its pre-F2 level.
An example of how this might work in practice would mean that when evaluating the
cost-effectiveness of a new medicine the PBAC would be required to take into account
the following for pricing purposes where nominated by the sponsor:
- an F1 medicine previously linked to the F2 clinical comparator, for example if the F2 comparator was part of an F1 price reference group prior to moving to F2, the price of another product in that F1 price reference group would be used in the evaluation; or
- where there is no equivalent F1 comparator, the price of the F2 comparator prior to its exposure to the mandatory and disclosure-based F2 price cuts resulting from the introduction of the two formularies and the price disclosure policy; or
- a current F1 medicine that is likely to become the market leader in clinical practice in the future, instead of the current F2 comparator, and thus can be used for pricing purposes in the evaluation.
It should be noted that this option would apply to both cost-minimisation and cost-effectiveness
analysis.
Strengths: This option quarantines F2 pricing effects like Option (a) but in addition allows
the PBAC to establish the relative clinical effectiveness of a medicine against all
currently listed medicines regardless of their F1 or F2 status, consistent with current
guidelines. This option also allows companies the flexibility to propose an equivalent
F1 price if they so choose, rather than it being an automatic requirement.
Weaknesses: This option and may increase the administrative complexity of any solution, as more
than one comparator may be involved, including the establishment of guidelines around
which F1 comparator should be used in pricing. The PBAC will be required to make its
decision based on a secondary analysis for pricing scenarios, possibly increasing
the administrative workload. The separation of the F1 and F2 formularies is compromised
at the pricing level, but not clinical level.
C) Use of F1 comparator for pricing purposes in certain circumstances
A variant to b) is to allow the use of an F1 price for pricing purposes, but for only
some new medicines based on a list of criteria. This would permit the Government to
exclude some new medicines with an F2 comparator having an F1 price adjustment on
the basis that they provide no additional benefit to the Australian community over
and above those products already listed in F2. A series of exclusion criteria could
be developed by the Government in consultation with industry. Variants to this option
include developing such criteria for including such medicines for F1 price adjustment, rather than the basis of exclusion from F1
price adjustment.
These criteria, whether used to exclude or include medicines for F1 price treatment,
could include a range of factors designed to ensure that patients did not miss out
on a range of new medicines in the future. As already flagged, F1 price treatment
occurs when a new medicine with an F2 comparator on clinical grounds is compared against
an equivalent F1 price for pricing purposes. When deciding whether to exclude/include
a medicine for such treatment, the Government may seek PBAC advice on the relevant
new medicine. Factors the PBAC might be required to consider, on a case by case basis,
in providing advice to the Government include:
- Whether the medicine is cost-effective – a medicine that PBAC has agreed is cost-effective at the F2 comparator price suggests the medicine has some significant improvement in patient outcomes over its comparator
- Even where a company is making a cost-minimisation submission for a new medicine (ie. ‘no worse than’ an F2 comparator), factors that should be considered include:
- The range and choice of alternative treatment options available to patients and health professionals for the disease area and whether the listing of the new medicine enhances that choice that results in an improved outcome for all or some patients
- Whether the medicine is a new mechanism of action, introduces a novel delivery action, a new pharmacological class or provides some other innovation of benefit that improves the outcome for the patient
- Whether there are other medicines already available that are interchangeable at the patient level with the proposed new medicine
- The extent to which the medicine provides a unique treatment, safety advantage or effectiveness advantage at least in some patients
- The extent to which formulations of the medicine can be uniquely used where other formulations cannot be used to benefit a sub-population of patients
- The extent to which patients, or a sub-group of patients, will be disadvantaged if the new medicine is not listed on the PBS
These criteria might be conveyed to the PBAC by the Minister in writing, as part of
a statement of intent confirming that the purpose of PBS reform is not to make it
more difficult for new medicines to be listed on the PBS in the future.
Strengths: As in b), but limits the use of F1 pricing of F2 comparators to medicines that provide
improvement in patient treatment. Does not provide higher pricing to medicines with
no additional benefit to patients or sub-groups of patients. While still at odds with
current cost-effectiveness practice it seeks to regularise the flexibility that is
inherent in the current PBAC decision making process, where PBAC consider it appropriate.
Weaknesses: While less at odds with cost-effectiveness processes than options a) or b), option
c) this is still at odds with cost-effectiveness analysis. Criteria need to be developed
not only of which F1 comparator should be used in pricing, but which new medicine
submissions with an F2 comparator should be given this treatment. The more prescriptive
the list of considerations, the more likely existing PBAC flexibility will be restricted.
Implementation aspects (DoHA & MA)
There are a range of issues that need to be considered should any one of the above options be implemented:
- Mandatory/discretionary consideration by PBAC of a new F1 medicine application
The extent to which PBAC’s ability to use its existing flexibility when considering the appropriate comparator will be constrained if mandatory requirements are imposed under options (a) or (b). PBAC currently has some flexibility in decision making which provides it and companies with a degree of room to move. A mandatory approach, namely that PBAC is required to use an F1 comparator or price, while providing additional certainty to companies in putting their new medicines forward for listing would limit this flexibility.
- Timing issues
Ideally, if a decision were made to adopt one of the options above as a solution for managing any potential impact of comparator price reductions, it would need to be put into effect as soon as practicable. Although it is conceivable that some F2 price reductions may have already influenced the comparator price for new medicine applications (eg. 12.5% cuts when a medicine moves from F1 to F2 prior to August 2008), in all likelihood the impact will not start to become apparent until after 1 August. The ability to have a solution in place in time is influenced by the extent to which that solution needs to be considered by Government and its administrative complexity.
- Cost
Each of these options may cause the PBS to cost more than would otherwise be the case if the impact of F2 comparator price reductions were allowed to flow fully on to the setting of the initial list price for new F1 medicines. However, this will need to be balanced against the possibility that there may be some new F1 medicines that may not be listed on the PBS or whose listing on the PBS may be somewhat delayed (if a company also has a price at which the new medicine would not be cost effective).
- How to effect any change
Implementing any of these options could be achieved through administrative process, following a Government decision about any policy change and resultant cost impact. While PBAC already has the flexibility to use alternate comparators at its discretion, mandating or restricting the type of comparator that can be used will require the development of clear administrative guidelines. A legislative approach may provide greater certainty to companies and PBAC on how medicines will be assessed, but requires lead time, is subject to Parliamentary approval and importantly compromises flexibility for both the PBAC and for companies. It is also likely that a non-legislative route will facilitate quicker implementation and while it could present problems of interpretation allows for flexibility and ease of updating as changes are required.
- Consultation with other stakeholders
The Government has made clear that it expects any initiatives emerging out of AMWG will require consultations with affected stakeholders before implementation. MA agrees with and supports this approach.
Conclusion
Through its examination of the comparators issue, the AMWG has confirmed that, in the future, there will be new drugs considered for listing in F1 which have an F2 comparator which has had a statutory price reduction. There is disagreement within the AMWG, however, as to the impact of this comparison.
MA believes that this is an issue which should be addressed by the application of one of the options discussed in the paper, with a preference for option a) Use of an F1 comparator. DoHA believes that the flexibility in the consideration of applications by PBAC is sufficient to handle any situations that may arise, including F2 comparators which have had a statutory price reduction. (It should be noted that while the two parties comprising the AMWG disagree on this point, this is not a reflection on the joint work conducted on this issue, which has been undertaken cooperatively and in the spirit in which the AWMG was formed.)
Next steps
DoHA and MA have agreed that each will separately brief the Minister for Health and Ageing on the likely impact of F2 comparator price reductions on new medicines seeking PBS listing and management options.
If the Minister requires further work DoHA and MA will work together, in consultation with any other parties the Minister indicates, to respond to the request.
References
1. AMWG, Agenda Paper 3.1, 18 December 2007. Comparator price reductions issues paper.
2. Medicines Australia, Agenda Paper 3.2, 18 December 2007. PBS Reform: Choice of
Comparator – Summary of Medicines Australia Position.
3. AMWG, Agenda Paper 3.3, 18 December 2007. Impacts of F2 on new F1 medicine pricing
– Meeting notes re preliminary options.
4. Medicines Australia, November 2007, MA member company survey – examples of future
F2 comparators for yet-to-be-considered F1 medicines – next five years.
Appendices
Appendix A – PBS Reform
In November 2006, the Government announced a number of changes to the PBS to protect
patients from higher out of pocket costs, get better value from market competition
among brands of generic (off-patent) medicines and recognise the importance of world-class
life-enhancing drugs to patients. The main change is in the way that the Government
prices medicines that are operating in a competitive market. These medicines will
take a series of price drops, and eventually will move to a system where the price
they are being sold for will reflect the price that the Government pays.
The New Formularies
From 1 August 2007, PBS medicines have been divided into two separate groups (‘formularies’), each subject to different pricing arrangements:
- F1 for medicines where there is only a single brand listed. F1 contains both on patent and off patent medicines that are not substitutable with other brands or medicines.
- F2 for multiple brand medicines, and single brand medicines interchangeable with a multiple brand medicine.
F2 is further divided into:
- F2A for multiple brand medicines with low levels of price competition; and
- F2T for multiple brand medicines with high levels of price competition.
Reference pricing
Reference pricing links the price of a medicine to the price of other medicines that provide a similar health outcome. It will continue for F1 medicines in reference price groups and for F2 medicines that belong to groups of medicines that are interchangeable between patients. If a price change occurs for one of these medicines, this will flow to the others.
Statutory Price Reductions
The requirement for a 12.5% price reduction when the first new brand of a medicine is listed on the PBS is continuing. There are no statutory price reductions for F1 medicines and existing price linkages are retained within F1. F2 medicines will experience statutory price reductions from 1 August 2008 as follows:
- F2A medicines will begin a series of three annual price reductions of 2%;
- F2T medicines will have a single 25% price reduction (phased over the remaining patent life for interchangeable patented medicines in therapeutic groups).
Price reductions no longer flow between medicines listed on different formularies. This represents a significant benefit for sponsors of many F1 drugs, which will no longer experience price reductions as a result of generic competition for medicines in the same reference priced group. In addition, Government and taxpayers will benefit from lower prices for multiple brand medicines in F2. Such price reductions may provide headroom for the listing of cost-effective new medicines.
Appendix B – Description of Price Disclosure
Under the 2006 PBS Reforms, the Government will over time move to a system of price disclosure, where the price that the Government pays will reflect the actual price at which the medicine is being sold.
Price disclosure will be phased in for medicines that operate in a competitive market:
- For medicines where price competition is low (F2A), suppliers of any new brand listing from 1 August 2007 will agree to disclose its price as a condition of listing. Price changes based on disclosure will commence for these medicines from 1 August 2009.
- For those medicines where price competition is high (F2T), suppliers of any new brand listing from 1 January 2011 will agree to disclose its price as a condition of listing. Price changes based on disclosure will commence for these medicines from 1 August 2012.
When the first new brand of a single brand medicine is listed on the PBS that medicine will become subject to regular price reductions and price disclosure.
Appendix C – Cost effectiveness and the incremental cost effectiveness ratio (ICER)
Cost effectiveness assessment compares the price advantage sought by a sponsor to the net additional health gains conferred by the new drug over its main comparator, together with any cost implications arising from changes in the provision of health care resources. The main comparator is the current therapy that prescribers would most replace with the new drug in practice. The main comparator can be another PBS-listed drug or, where there is no existing effective drug therapy, placebo for standard medical management of the condition.
The preferred metric for describing the overall changes in health outcomes is the number of Quality Adjusted Life Years (QALYs) gained. This summarises the effects of a health intervention on both survival and quality of life.
In calculating the costs associated with listing a drug on a cost-effectiveness basis, a wide range of costs (and cost offsets) can be taken into account beyond drug costs. These can include diagnostic, medical, hospital, residential age care and allied health services costs. For the sake of simplicity, the discussion and examples below focus on the drug costs of the new drug and its main comparator only and not any other sources of costs and cost offsets.
The ratio of additional costs to additional health gains is known as the Incremental Cost Effectiveness Ratio (ICER), which is simplified below as:
ICER = ![]()
The ICER presents the extra cost for each additional QALY gained offered by the new drug therapy. The larger the ICER, the more “expensive” is the additional health gain and the less favourable the economic credentials are for a PBAC recommendation to list. Increasing the price advantage requested over the comparator increases the value of the ICER. Increasing the additional QALY gained decreases the value of the ICER.
While there is no single maximum ICER value set by PBAC – no “cost effectiveness threshold” – there is evidence that medicines are more likely to be recommended for listing with an ICER around $30,000 than with an ICER above $70,000.
Table 1 provides an example of a new drug with an ICER of $30,000. A $1,500 price advantage is requested for the new drug, and there is an additional health gain of 0.05 QALYs. In this simple example there are no non-drug costs or cost offsets to take into account.
| Comparator cost/year | $500 |
| New drug cost/year | $2,000 |
| Price advantage requested | $1,500 |
| Additional QALY gain/year | 0.05 |
| ICER | $30,000 |
Appendix D – Comparator Analysis (Medicines Australia)
MA provided a report on a review of 113 PBAC decisions from November 2005 to November
2006 through information available in public summary documents (PSDs). The analysis
indicated that for cost effectiveness submissions:
- the comparator was a F1 medicine or placebo in approximately 85% of cases; and
- the comparator was a F2 medicine in approximately 15% of cases.
In commissioning this analysis, the MA representatives advised that they anticipated
the impact of price reductions could be greatest for:
- The case of a new high cost medicine with a good QALY gain (eg. >0.1) where the comparator is also high cost but has moved to F2. In this case, the ICER may move from what is acceptable to one that exceeds what PBAC considers acceptable value for money.
- The situation where a modest price advantage is requested over a moderate to low priced comparator but the new drug’s QALY gain is low (eg chronic therapy in therapeutic areas such as the current TGP groups)
- A product of modest price that attempts to cost-minimise against a low priced comparator which has been subject to a 25% price cut.
By way of example, a range of scenarios presenting increases in the cost/QALY ratio as a result of a 25% price reduction are presented below. In this example, new high cost medicines that have a relatively high cost comparator (eg. some of the treatments for cancer) experience a significant increase in the cost/QALY ratio as a result of a 25% price reduction in their comparator (noting MA’s view that some F2 comparators are likely to see a greater than 25% price reduction as a result of PBS reform). MA notes previous DoHA analysis showing that a 50% reduction in the price of a comparator for a sample of existing medicines on the PBS shows an increase in the incremental cost-effectiveness ratio of between 0% and over 70% depending on the medicine, raising the possibility that some new medicines on the PBS now would not have been listed if their comparators had undergone PBS reform price reductions at the time.
| Pre-PBAC Reform | |||
| Price Adv of Drug | Price of Comparator | QALY gain | |
| Large 0.15 |
Small 0.04 |
||
| Large | Large $10,000 |
$33,333 | $125,000 |
| $15,000 | Small $1,000 |
$99,333 | $350,000 |
| Small | Large $1,000 |
$3,333 | $12,500 |
| $1,500 | Small $500 |
$6,667 | $25,000 |
| Post-PBAC Reform (25% price reduction) | |||
| Price Adv of Drug | Price of Comparator | QALY gain | |
| Large 0.15 |
Small 0.04 |
||
| Large | Large $7,500 |
$50,000 | $187,500 |
| $15,000 | Small $750 |
$95,000 | $356,250 |
| Small | Large $750 |
$5,000 | $18,750 |
| $1,500 | Small $375 |
$7,500 | $28,125 |
Appendix E – Comparator Analysis (Department of Health and Ageing)
DoHA looked at the comparator used for each major drug submission recommended for
listing by PBAC between March 2004 and August 2006. This focus on the subset of all
submissions which were recommended by PBAC was justified because it excluded submissions
rejected by PBAC for reasons which would not be affected by statutory price reductions.
During that period PBAC gave positive recommendations for 106 major submissions:
- 44 (42%) listed on a cost effectiveness basis; and
- 62 (58%) listed on a cost minimisation basis.
Diagram 1 shows what proportion of submissions recommended by PBAC on a cost-effectiveness basis used another medicine as the comparator (55%) in their economic analysis, and what proportion used placebo/standard medical care (45%). It further breaks down the PBS-listed comparators into the formularies that those drugs have since been assigned. Seven per cent (n=3) of the total sample used drugs now listed on the F2T formulary as comparators.
Diagram 1
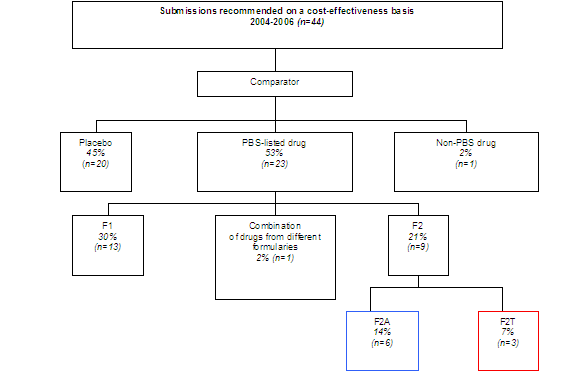
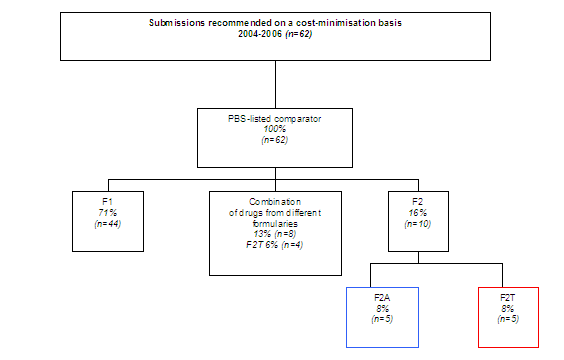
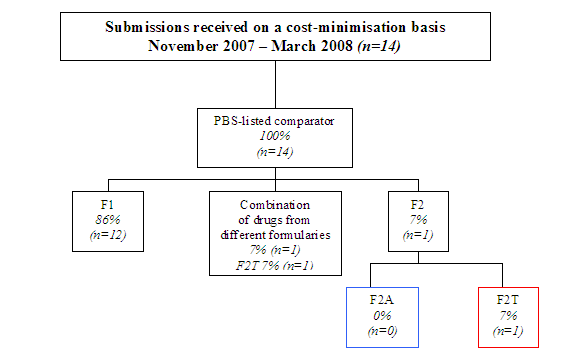
Diagram 2 shows what proportion of submissions recommended by PBAC on a cost-minimisation basis used comparators on each formulary. All cost minimised drugs have PBS-listed comparators. Eight per cent (n=5) of the total sample used comparators now listed on the F2T formulary.
Diagram 2
Effect of a price reduction for a F2T comparator on cost minimised drugs
Cost minimised drugs list at the same price as the comparator. However, the creation of the formularies prevents a new single brand drug from having its price linked to the comparator over time. As a result of the PBS reforms, for products that seek listing on the basis of cost-minimisation analysis where the comparator is a F2 product, it may be problematic for a company to list the product (given global floor price issues) at the reduced price of the F2 comparator. In order minimise the impact of a statutory price reduction, a sponsor may attempt to support listing on a cost effectiveness basis by seeking a tighter restriction to a subgroup of patients unable to benefit from the comparator for sound clinical reasons.
Effect of a price reduction for a F2T comparator on the ICER
The simplified and illustrative analysis below attempts to isolate the difference
in the ICER before and after a 25% price reduction is applied to the comparator drug.
The analysis is qualified by the following assumptions, which limit the generality
of the results.
Simplifying assumptions for analysis
1. All other factors relevant to cost effectiveness assessment, including health and safety benefits (QALY gain), and the price sought by the sponsor of the new drug, are held constant before and after the statutory price reduction is applied. In practice a number of options will be available for the sponsor after a comparator price reduction, including reducing the price sought or attempting to identify a subgroup of the patient population for which acceptable cost effectiveness may be achievable at a larger price advantage.
2. The full costs of both the comparator and the new drug are due to the medicines
only with no non-drug costs or cost offsets. A more complex and realistic analysis
would take these additional cost factors into account. For example, if half the cost
of treatment with the comparator is due to necessary tests and procedures (which will
be unaffected by statutory price reductions) then, in effect, a 25% reduction in the
comparator drug price will result in only a 12.5% reduction in the overall cost of
therapy involving the comparator.
Summary conclusions from analysis
- The change in the ICER following a reduction in the price of the comparator is determined by the size of the price advantage requested for a new drug. The smallest changes occur when the price advantage requested is large (multiples of the comparator price). Larger changes occur when the price advantage requested is small (some fraction of the comparator price).
- The dollar amount by which the ICER increases as a result of a reduction in the comparator price is less when the additional health gain offered by a new drug is large.
Analysis
On the assumption that the sponsor would seek the same price for its new drug before and after a reduction in the price of the comparator, the price reduction impacts the ICER by increasing the extent of the price advantage requested.
Tables 1 and 2 illustrate this below. In each case, the sponsor seeks a price of $2,000 per year, with an additional QALY benefit of 0.05. In Table 1, this represents a 300% increase over the comparator’s price of $500 before the statutory reduction. The ICER increases by 8% from $30,000 to $32,500 after the comparator’s price is reduced.
In Table 2, the new drug’s price is only 33% higher than the $1,200 price of the comparator before the statutory reduction. This means that, at a value of $16,000, the resulting ICER is less than in Table 2 before a reduction in the price of the comparator. After the comparator’s price is reduced, this ICER increases by 38% to $22,000.
|
Table 1 |
Table 2 |
||||
| Large price advantage requested | Pre-25% | Post-25% | Small price advantage requested | Pre-25% | Post-25% |
| Comparator cost/year | $500 | $375 | Comparator cost/year | $1,200 | $900 |
| New drug cost/year | $2,000 | $2,000 | New drug cost/year | $2,000 | $2,000 |
| Price advantage requested | $1,500 | $1,625 | Price advantage requested | $800 | $1,100 |
| Price increase (%) | 300% | 433% | Price increase (%) | 33% | 122% |
| Additional QALY gain/year | 0.05 | 0.05 | Additional QALY gain/year | 0.05 | 0.05 |
| ICER | $30,000 | $32,500 | ICER | $16,000 | $22,000 |
| Increase in ICER (%) | 8% | Increase in ICER (%) | 38% | ||
Within the limitations of the analysis identified above, the extent of the additional QALY gain offered by a new drug plays no role in determining the percentage change in the ICER resulting from a comparator price reduction. However, a larger QALY gain will mean that the dollar value of the ICER increase is smaller. This can be seen in Tables 3 and 4 below, which show examples differing only in the additional QALY gain offered by the new drug. In each case, the sponsor seeks a price for the new drug of $5,000 per year, which is a 400% increase over the comparator’s price of $1,000 before the statutory reduction.
In Table 3, the additional QALY gain is 0.05, resulting in an ICER before the comparator price reduction of $80,000. In Table 5, the additional QALY gain is 0.1, giving an ICER before the comparator price reduction of $40,000. In each case the ICER increases by 6% after the comparator price is reduced.
However, although the percentage increases in the ICER are the same, the dollar value increases by $5,000 for the drug with the lower QALY gain (Table 3) and only $2,500 for the drug with the higher QALY gain (Table 4).
As ultimately it is the dollar increase in the ICER that is most relevant to assessing incremental cost effectiveness, this analysis shows that the advantage that a new drug offering large additional health gains has in achieving acceptable cost effectiveness is maintained in an environment where comparator prices are reduced.
|
Table 3 |
Table 4 |
||||
| Large price advantage requested |
Pre-25% |
Post-25% |
Small price advantage requested |
Pre-25% |
Post-25% |
| Comparator cost/year |
$1,000 |
$750 |
Comparator cost/year |
$1,000 |
$750 |
| New drug cost/year |
$5,000 |
$5,000 |
New drug cost/year |
$5,000 |
$5,000 |
| Price advantage requested |
$4,000 |
$4,250 |
Price advantage requested |
$4,000 |
$4,250 |
| Price increase (%) |
400% |
567% |
Price increase (%) |
400% |
567% |
| Additional QALY gain/year |
0.05 |
0.05 |
Additional QALY gain/year |
0.1 |
0.1 |
| ICER |
$80,000 |
$85,000 |
ICER |
$40,000 |
$42,500 |
| Increase in ICER (%) |
6% |
Increase in ICER (%) |
6% |
||
Table 5 below shows the percentage increase in the ICER resulting from a 25% comparator price reduction for a selection of annual costs for new drugs and for comparators (listed at their prices before this statutory reduction is applied). (The percentage increase in the value of the ICER following a 25% comparator price reduction can be determined quickly by dividing 25% by the percentage price increase requested (before the comparator price reduction and for equal durations of therapy). For example, if the new drug price sought is 400% of the comparator price, the ICER increases by 6.25% - this corresponds to the examples in Tables 3 and 4.) Note that the current average cost of PBS items on the F2T formulary is approximately $270 per annum.
| % increase in ICER | New drug price sought | ||||||||
| $200 | $300 | $500 | $1,000 | $2,000 | $5,000 | $10,000 | $15,000 | ||
| Comparator price (before-reduction) | $100 | 25% | 13% | 6% | 3% | 1% | 1% | 0% | 0% |
| $200 | 50% | 17% | 6% | 3% | 1% | 1% | 0% | ||
| $300 | 38% | 11% | 4% | 2% | 1% | 1% | |||
| $500 | 25% | 8% | 3% | 1% | 1% | ||||
| $1,000 | 25% | 6% | 3% | 2% | |||||
| $2,000 | 17% | 6% | 4% | ||||||
| $5,000 | 25% | 13% | |||||||
| $10,000 | 50% | ||||||||
Appendix F – Further Comparator Analysis November 2007 – March 2008
Diagram 1
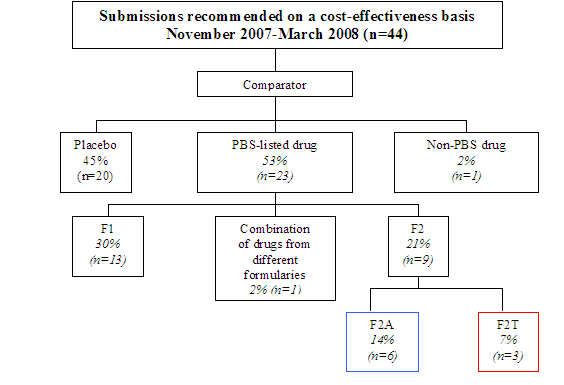
Diagram 2
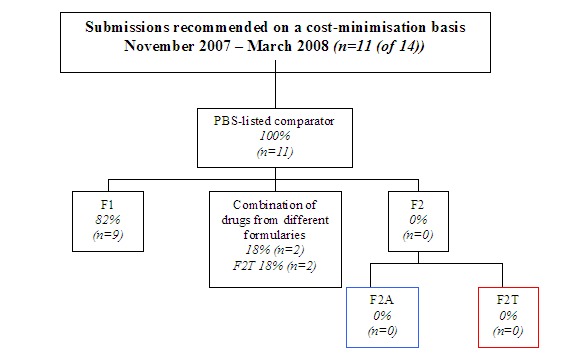
Diagram 3
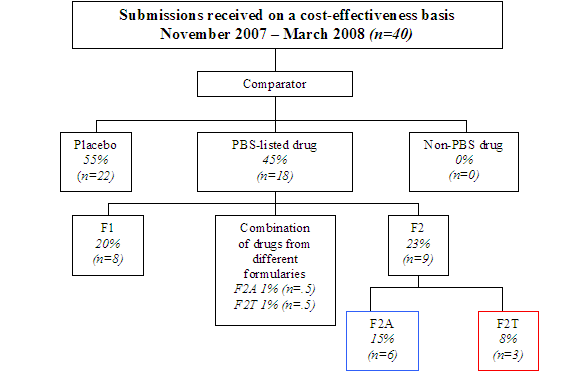
Diagram 4
Appendix G – PBAC evaluation of new listings
The economic basis of a PBAC recommendation usually follows one of two approaches.
- A cost-minimisation approach applies where there are insufficient gains in health outcomes to justify a higher price for the proposed drug over currently listed alternatives. Drugs seeking listing on a cost-minimisation basis simply need to demonstrate that, therapeutically, they are at least as effective and safe as (“no worse than”) a comparator drug already listed on the PBS. Cost-minimisation results in listing at the same price as the comparator based on equi-effective doses.
- In contrast, a cost-effectiveness approach allows a proposed new drug to be listed with a price advantage over its comparator, provided the extent of this price advantage can be justified by improved health outcomes (often summarised as an additional Quality Adjusted Life Years (QALY) gain) and/or cost offsets resulting from changes in the provision of other health care resources. The ratio of additional costs to additional health gains is known as the Incremental Cost Effectiveness Ratio (see Appendix C).
In addition to the results of economic analysis, PBAC takes other relevant factors into account in the process of deciding whether to recommend that a drug be listed. These include:
- the extent of uncertainty relating to the claims made about cost and heath outcomes;
- the total annual costs to the PBS of implementing the listing;
- the extent to which a restriction can be constructed which satisfactorily distinguishes uses which are acceptably cost-effective to justify subsidy from uses which are not cost-effective;
- the scope for usage of the drug beyond any restriction for subsidy;
- the severity of the condition being treated;
- the affordability of the medicine to the patient in the absence of a subsidy; and
- the availability of other effective interventions for the condition.
The decision whether or not to recommend listing requires an overall assessment of all relevant factors – combining a consideration of the results of the economic analysis and other relevant factors such as those listed above.
Appendix H - Examples of how options a) to c) might work in listing new medicines on the PBS
a) Automatic use of F1 comparator
A company submits new anti-depressant therapy for listing on the PBS. While its clinical
comparator might be an existing anti-depressant treatment in F2, the new therapy is
required to have a similar or alternative anti-depressant therapy in F1 for clinical
and economic evaluation and all evaluation is done against this alternative F1 comparator.
Where there is no alternative F1 anti-depressant treatment, the new anti-depressant
is evaluated for cost-effectiveness against placebo for standard medical management.
b) Use of F1 price for pricing purposes only
A company makes a submission to list a next generation diabetes medicine, but its clinical comparator is in F2. While the medicine is a significant incremental improvement over existing therapies, given the price reductions of the F2 comparator, the medicine is unable to demonstrate cost-effectiveness at the F2 comparator’s reduced price – given the new differential between the new diabetes medicine and the older one in F2, the medicine is now not cost-effective in spite of being a clinical improvement. When the F2 comparator’s older F1 price is used, however, the new diabetes treatment is cost-effective. In this case, the company may elect to submit the F2 comparator’s pre-PBS reform price for pricing purposes and cost-effectiveness calculations.
In another example, a new treatment for prostate cancer is cost-effective over an
older F2 comparator at the F2 comparator price; that is, the treatment is an improvement
for prostate cancer patients. The F2 comparator may recently have moved into F2 and
undergone price reductions as a result of PBS reform, leaving behind one or more other
prostate cancer treatments in a reference pricing group in F1 which, although other
treatments for prostate cancer, are not the clinical comparator. Prior to entry into
F2, the comparator may have been priced at the same level as other prostate cancer
treatments in F1. In this case, the equivalent F1 price of another prostate cancer
medicine will be substituted in the place of the F2 comparator’s price for pricing
purposes.
c) Use of F1 comparator for pricing purposes in certain circumstances
A company is seeking listing for a new treatment for psoriasis against an F2 comparator. While cost-minimised for clinical purposes, PBAC may agree that the treatment does provide a benefit in terms of patient choice or an additional treatment option, and therefore should have an equivalent F1 price for pricing purposes. In this example, no equivalent F1 reference pricing group may exist, hence the price of the F2 comparator prior to its move into F2 is used for pricing purposes.
In a further example, a company is seeking to list a new ACE inhibitor medicine. The submission is a cost-minimisation submission against an existing ACE inhibitor in F2, offers no additional patient benefit than existing treatments and is considered interchangeable with several other existing ACE inhibitors, all in F2. In this circumstance, the PBAC may recommend that the new ACE inhibitor not be given an equivalent F1 price.




