Infliximab, powder for I.V. infusion, 100 mg, Remicade®, March 2007
Public summary document for Infliximab, powder for I.V. infusion, 100 mg, Remicade®, March 2007
Page last updated: 29 June 2007
Printable Version of Infliximab powder for I.V. infusion 100 mg (Remicade) (PDF 136 KB)
Public Summary Document
Product: Infliximab, powder for I.V. infusion, 100
mg,
Remicade®
Sponsor: Schering-Plough Pty Ltd
Date of PBAC Consideration: March 2007
1. Purpose of Application
The re-submission requested an extension to the Section 100
Authority Required listing to include the treatment of severe
refractory Crohn’s disease.
2. Background
Submissions requesting subsidy of infliximab for refractory
moderate to severe Crohn's disease have been considered at three
PBAC meetings: June 2000, December 2000, and September 2001. On
each occasion the PBAC rejected the request for subsidy.
3. Registration Status
Infliximab is registered by the Therapeutic Goods Administration
(TGA) for the following indications:
Rheumatoid Arthritis in Adults: Remicade, in
combination with methotrexate, is indicated for the reduction of
signs and symptoms and prevention of structural joint damage
(erosions and joint space narrowing) in: 1.patients with active
disease despite treatment with methotrexate, 2.patients with active
disease who have not previously received methotrexate. Remicade
should be given in combination with methotrexate. Efficacy and
safety in Rheumatoid Arthritis have been demonstrated only in
combination with methotrexate.
Ankylosing Spondylitis: Remicade is indicated for
the reduction of signs and symptoms and improvement in physical
function in patients with active disease.
Crohn's Disease: Remicade is indicated for the
treatment of moderate to severe Crohn's disease, to reduce the
signs and symptoms and to induce and maintain clinical remission in
patients who have an inadequate response to conventional
therapies.
Refractory Fistulising Crohn's Disease: Remicade
is indicated for reducing the number of draining enterocutaneous
and rectovaginal fistulas and maintaining fistula closure.
PSORIATIC ARTHRITIS: Remicade is indicated for the treatment of the
signs and symptoms of active psoriatic arthritis in adults where
previous response to disease-modifying anti-rheumatic drugs
(DMARDS) has been inadequate.
Psoriasis: Remicade is indicated for the treatment
of adult patients with moderate to severe plaque psoriasis for whom
phototherapy or conventional systemic treatments have been
inadequate or are inappropriate. Safety and efficacy beyond 12
months have not been established.
Infliximab was approved by the TGA for maintenance treatment of
moderate to severe Crohn’s disease in July 2003.
4. Listing Requested and PBAC’s View
Section 100 Authority Required Listing (Highly Specialised
Drug)
Public and private hospital authority required
Initial treatment by a gastroenterologist of adult patients with
severe Crohn’s disease who satisfy the following criteria;
and, who have signed a consent form, authorised by Medicare
Australia, to indicate acceptance that PBS subsidy of infliximab
will cease if the required response criteria are not met. Patients
must meet the following initiation criteria:
- Confirmed Crohn’s disease, defined as standard clinical, endoscopic and/or imaging features, including histological evidence, with diagnosis confirmed by a gastroenterologist, and
- Severity of disease activity that results in a Crohn’s Disease Activity Index Score (CDAI) > 220 or by clinical discretion if the patient has an ileostomy or has had a colectomy, and
- Failed an adequate trial of conventional therapy; unless contraindicated, patients must be unable to tolerate, or be unresponsive to:
- A tapered course of steroids, starting at > 40 mg prednisolone (or equivalent), over a six week period, and
- Immunosuppressive therapy, including azathioprine or 6-mercaptopurine or methotrexate at optimal dosage for > 3 months.
With failure of conventional therapy defined as having a CDAI >
220 despite an adequate trial of conventional therapy defined
above.
The authority application must be in writing and must include the
information used to determine the patient’s eligibility under
the criteria above. Dose and maximum quantity for initial course of
infliximab: 3 doses at 5 mg/kg body weight per dose at weeks 0, 2
and 6.
Public and private hospital authority required
Continuing treatment by a gastroenterologist, or consultant
physician in consultation with a gastroenterologist, of adults with
severe Crohn’s disease who have received three doses of
PBS-subsidised treatment with infliximab and who, at the time of
application have achieved or sustained a response to treatment with
infliximab. Response is defined as a reduction in Crohn’s
Disease Activity Index Score (CDAI) of > 70 points from baseline
CDAI.
The first application for continuing treatment should be made
following administration of the first three infusions; (i.e.
approximately 12 weeks from the commencement of treatment). Second
and subsequent applications for continuing treatment must be made
in writing and posted to Medicare Australia no less than 2 weeks
prior to the date the next dose is scheduled, to ensure continuity
of treatment.
A maximum of 24 weeks of treatment (3 infusions) with infliximab
will be authorised under this criterion. At the time of the
authority application, medical practitioners should request the
appropriate number of vials, based on the weight of the patient, to
provide sufficient for a single infusion at a dose of 5 mg per kg.
Up to a maximum of 2 repeats will be authorised. No applications
for increased repeats will be authorised. Where fewer than 2
repeats are initially requested with the authority prescription,
authority approvals for sufficient repeats to complete a maximum of
24 weeks of treatment may be requested by telephone.
Patients who fail to demonstrate or sustain a response to treatment
with infliximab for Crohn’s disease as specified in the
criteria for continuing treatment with infliximab will not be
eligible to recommence treatment with this drug within 12 months of
the date on which treatment was ceased.
Applications for PBS-subsidised treatment will not be authorised
for patients who have failed two PBS-subsidised courses of
treatment with infliximab.
Where re-treatment with infliximab after a break in PBS-subsidised
treatment with infliximab is being sought, the reason for and date
of cessation of the previous treatment course with infliximab must
be included in the application.
Public and private hospital authority required
Initial treatment – Grandfather listing (to be developed by
the Pharmaceutical Benefits Branch and Schering Plough)
Public and private hospital authority required
Continuing treatment – Grandfather listing (to be developed
by the Pharmaceutical Benefits Branch and Schering Plough)
See Recommendation and Reasons for PBAC’s
view.
5. Clinical Place for the Proposed Therapy
Infliximab would provide clinicians with a biological Disease
Modifying Anti-Rheumatic Drug (bDMARD) therapy for Crohn’s
patients who continue to have active disease despite optimal
treatment with conventional therapies including surgery.
6. Comparator
The nominated comparator was placebo as add on to immunosuppressive
agents.
7. Clinical Trials
The submission presented an unadjusted indirect comparison of
infliximab 5 mg/kg induction and maintenance treatment (from the
newly presented trial ACCENT 1) compared to placebo (from the
previously presented trial T16, excluding patients with
fistulae).
The trials published at the time of the submission are tabulated
below:
| Trial/First author | Protocol title/Publication title | Publication citation |
| Targan et al. (1997) (T16) | A short-term study of chimeric monoclonal antibody cA2 to tumour necrosis factor (alpha) for Crohn’s disease. | NEJM 337 (15): 1029-1035. |
| Hanauer et al. (2002) | Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. | Lancet 359 (9317): 1541-1549. |
The original primary trial endpoints (ACCENT 1: loss of response to
week 54; T16: clinical response at week 4) were not the primary
endpoints used in the submission. Clinical response (CDAI score
≤70 from baseline) at week 14 was the new primary endpoint for
the submission re-analysis, conducted using individual patient
data. The Pre-Sub-Committee response advised the CDAI outcome of a
reduction of 70 points or more was defined a-priori in T16 and
ACCENT 1 and was agreed previously at a stakeholder meeting in
2002.
8. Results of Trials
Week 14 and 54 results of the unadjusted indirect re-analysis are summarised in the
table below.
Clinical response and remission at week 14 – IPD re-analysis in the re-submission
based on CDAI score
| ACCENT I 5mg/kg N=192 | T16 Placebo N=19 | Estimated effect (induction + active maintenance 5 mg/kg vs. placebo | |||
| OR (95%CI) | ARD (95%CI) | p value | |||
| Week 14 | |||||
| Response (%ITT a) | 99 (51.6) | 0 (0.0) | 41.5 (2.47, 697.17) | 51.6 (44%, 59%) | <0.0001 |
| Remission (%ITT a) | 67 (34.9) | 0 (0.0) | 20.98 (1.25, 352.85) | 34.9 (28%, 42%) | 0.0006 |
| Week 54 | |||||
| Response (%ITTa) | 63 (32.8) | 0 (0) | 19.12 (1.14, 321.48) | 32.8 (26%, 39%) | 0.0011 |
| Remission (%ITT a) | 44 (22.9) | 0 (0) | 11.69 (0.69, 197.45) | 22.9 (17%, 29%) | 0.0155 |
At week 14, 51.6% of the patients in the infliximab 5 mg/kg treatment arm, compared
with 0.0% of patients in the placebo arm (p<0.0001), were considered to have a clinical
response. Clinical remission was observed in 34.9% and 0.0% (p=0.0006) of the infliximab
5 mg/kg and placebo arms, respectively.
The PBAC also considered evidence in the ACCENT I publication (Hanauer et al 2002)
which presented the results for patients randomised at week 2 as responders. See figures
below.
Kaplan Meier estimate of the proportion of patients who had not lost response by time
interval through week 54: ACCENT I (only patients randomised as responders at week
2)a
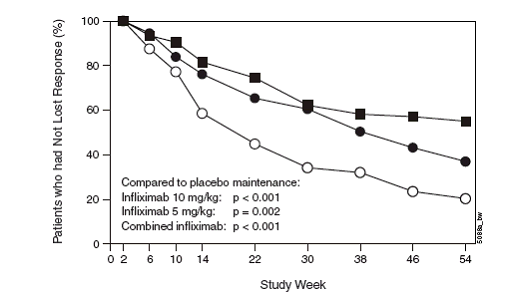

a: P-values for comparing Remicade active maintenance groups (each and combined) versus
placebo maintenance group were log-rank tests. Patients included only those randomised
as responders (as per study report Fig 14). NB. even placebo maintenance patients
had received infliximab induction dose.
Incremental effect of multiple doses as compared to single dose:
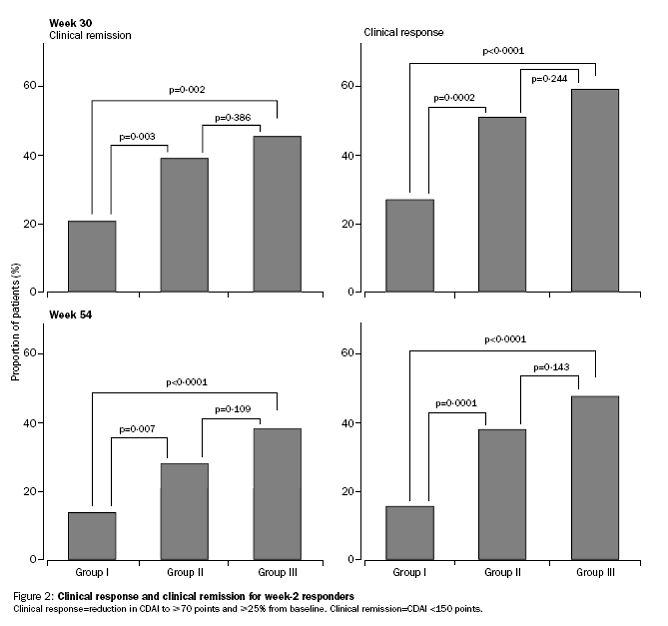
Median CDAI and IBDQ scores to week 54.
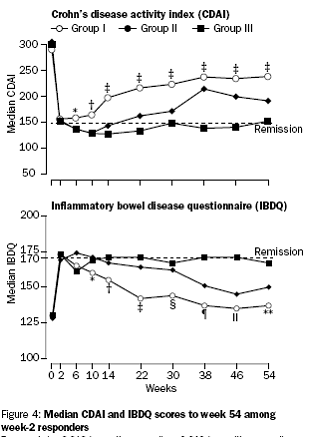
The PBAC noted that the evidentiary basis for the re-submission’s request for listing
was weak, because the re-submission did not provide direct data comparing a maintenance
regimen of multiple doses of infliximab with placebo, which was what the submission
requested. The indirect comparison was hindered by differences in the populations
enrolled in the placebo arm of T16 trial and the 5 mg/kg arm of ACCENT 1 trial. On
the other hand, the data provided helped in identifying the relevant benefits and
harms of short-term versus long term use of infliximab.
Adverse events data was derived directly from the included trials. No individual patient
data was available for re-analysis of adverse events. Infliximab was associated with
a greater frequency of adverse events than placebo (65% versus 24%). Mild infusion
reactions were relatively common (6.1%), however, one serious allergic reaction occurred.
The incidence of delayed hypersensitivity reactions was low (2.6%). Post-marketing
surveillance continued to observe low rates of malignancy, delayed hypersensitivity
reactions and auto-immune conditions.
9. Clinical Claim
The submission claimed that infliximab, through multiple
injections, has significant advantages over placebo but has more
toxicity.
See Recommendation and Reasons for PBAC’s
views.
10. Economic Analysis
An updated preliminary economic evaluation using a
cost-effectiveness approach was presented.
The trial based incremental cost effective ratio (ICER) was
estimated to be in the range of $15,000 - $45,000 for response and
remission.
An updated modelled economic evaluation adopting a cost utility
approach was presented.
The base case modelled incremental cost/QALY was estimated to be in
the range of $45,000 - $75,000.
The PBAC noted uncertainties associated with the model.
11. Estimated PBS Usage and Financial Implications
The likely number of vials sold/year was estimated to be <
10,000 in Year 2008-2009.
The financial cost/year to the PBS was estimated to be in the range
of $10 - $30 million in Year 2007-2008.
12. Recommendation and Reasons
The PBAC recommended the listing of infliximab for the treatment of
patients with severe Crohn’s disease (Crohn’s Disease
Activity Index ≥ 300) or patients with an ileostomy or colectomy
due to Crohn’s disease on the basis of high and acceptable
cost-effectiveness compared to placebo. Acceptable
cost-effectiveness was demonstrated at a dose of 5 mg/kg infliximab
for three doses (weeks 0, 2 and 6) and when continuation of
treatment beyond three doses was determined by remission (CDAI ≤
150) at approximately 12 weeks from the commencement of treatment.
The PBAC recommended that where a response to infliximab was not
demonstrated patients would not be eligible to recommence treatment
with infliximab within 12 months of the date on which the treatment
ceased.
The PBAC noted that listing had been sought for patients with
moderate to severe disease (CDAI ≥ 220) based on an unadjusted
indirect comparison of infliximab 5 mg/kg induction and maintenance
treatment (ACCENT 1) compared to a subset of the small placebo arm
of the T16 trial (n=19). The indirect comparison was hindered by
differences in the populations enrolled in two arms of the
different the trials, however, the data provided did help in
identifying the relevant benefits and harms of short-term use
versus long-term use of infliximab.
While there are biologically plausible concerns of infection,
malignancy and auto-immune diseases associated with the use of
infliximab in Crohn’s disease, the PBAC noted post-marketing
surveillance to date has shown low levels of malignancy and
auto-immune conditions.
The PBAC noted there were concerns with the modelled economic
evaluation. The PBAC also noted the evidence provided in the agenda
papers and at the hearing in relation to the clinical need for the
drug in this patient group, and the potential for patients to
benefit in terms of quality of life (QoL).
Overall, the PBAC considered the cost/QALY of between $45,000 -
$75,000 was high and uncertain. However, by limiting infliximab to
a more severe population and requiring a demonstration of remission
for continuing therapy, the PBAC considered that the incremental
cost-effectiveness ratio would improve sufficiently (albeit by an
unknown amount) to allow a positive recommendation. The PBAC
recommended that those Crohn’s disease patients with an
ileostomy or colostomy also be eligible because the severity of
their disease is underestimated by the CDAI and there is no
suitable alternative instrument to gauge the severity of their
disease in a way that can be correlated with the CDAI. However, the
PBAC would be pleased to receive further information from the
sponsor about the effect on the incremental cost-effectiveness
ratio of relaxing these restrictions to allow either PBS subsidy
for infliximab in less severe patients and/or continuation of
PBS-subsidised infliximab for those achieving a response rather
than a remission.
Recommendation
INFLIXIMAB, powder for I.V. infusion, 100 mg
Restriction: Restriction to be finalised
Maximum quantity: 1
Repeats: nil
13. Context for Decision
The PBAC helps decide whether and, if so, how medicines should be
subsidised in Australia. It considers submissions in this context.
A PBAC decision not to recommend listing or not to recommend
changing a listing does not represent a final PBAC view about the
merits of the medicine. A company can resubmit to the PBAC or seek
independent review of the PBAC decision.
14. Sponsor’s Comment
The sponsor commends the PBAC for its decision to recommend
Remicade for a select group of Crohn’s disease patients,
however believes that the criteria proposed by the PBAC are too
restrictive.
In the interest of patients with severe Crohn’s disease the
sponsor accepts the PBAC recommendation.
Schering-Plough views this listing as a positive first step to
assisting all patients with Crohn’s disease and will continue
to work with the Gastroenterological Society of Australia and the
Australian Crohn’s and Colitis Association towards making
Remicade available to a broader patient population.




