Clostridium botulinum type A toxin-haemagglutinin complex, lyophilised powder for I.M. injection, 500 units/vial, Dysport, November 2007
Public summary document for Clostridium botulinum type A toxin-haemagglutinin complex, lyophilised powder for I.M. injection, 500 units/vial, Dysport, November 2007
Page last updated: 29 February 2008
Public Summary Document
Product: Clostridium botulinum type A toxin-haemagglutinin complex, lyophilised powder
for I.M. injection, 500 units/vial, Dysport
Sponsor: Ipsen Pty Ltd
Date of PBAC Consideration: November 2007
1. Purpose of Application
The submission sought an extension to the Section 100 (BOTULINUM TOXIN PROGRAM) listing to include treatment of moderate to severe spasticity of the upper limb in adults following a stroke, as an adjunct to physical therapy. Spasticity is defined as a Modified Ashworth Scale (MAS) score ≥ 2 in at least 2 joints of upper limb.
2. Background
An application seeking PBS listing of clostridium botulinum type A toxin- haemagglutinin
complex for “treatment of spasmodic torticollis, either as monotherapy or as adjunctive
therapy to current standard care” was recommended at the December 2000 PBAC meeting.
An application to extend the Section 100 listing to include ‘treatment of dynamic
equinus foot deformity due to spasticity in ambulant paediatric cerebral palsy patients,
two years of age or older’, was recommended at the December 2001 PBAC meeting.
Applications to add the indication “treatment of spasticity of the arm in adults following
a stroke” were rejected at the September 2001 and September 2002 PBAC meetings because
of uncertainty over the extent of clinically relevant benefits and the resulting uncertain
cost-effectiveness.
3. Registration Status
Clostridium botulinum type A toxin- haemagglutinin complex is TGA registered for:
Spasticity of the upper limb in adults following a stroke (24 May 2001),
Spasmodic torticollis in adults (18 April 2000),
Dynamic equinus foot deformity due to spasticity in ambulant paediatric cerebral palsy
patients, 2 years of age or older (31 August 2001),
Blepharospasm in adults (6 May 2004),
Hemifacial spasm in adults (6 May 2004),
Moderate to severe glabellar lines in adults (17 October 2005).
4. Listing Requested and PBAC’s View
Section 100 listing
Botulinum Toxin Program
Clostridium Botulinum Type A Toxin-Haemagglutinin Complex
Treatment of moderate to severe spasticity of the upper limb in adults following a
stroke as an adjunct to physical therapy.
Spasticity defined as MAS ≥ 2 using modified Ashworth scale in at least 2 joints of
upper limb.
Maximum number of treatments to be authorised is 4.
For PBAC’s view, see Recommendation and Reasons.
5. Clinical Place for the Proposed Therapy
Clostridium botulinum type A toxin-haemagglutinin complex injection therapy treats spasticity by allowing the muscle to relax. The effects of Clostridium botulinum type A toxin- haemagglutinin complex are reversible and treatment provides a window of opportunity in which other treatments, such as physical therapies, can be used to regain muscle or joint function.
6. Comparator
The submission nominated standard management (physiotherapy, orthoses oral anti-spasticity drugs) of spasticity of the upper limb post-stroke, as the main comparator. This was previously accepted by the PBAC.
7. Clinical Trials
The submission presented a new clinical trial (Study 097) as key evidence: a prospective
phase IV, multicentre, placebo-controlled study that was conducted in six centres
in Australia. The submission also presented supportive studies, which were presented
in the previous submission, and supportive evidence from two meta-analyses (Francis
et al, 2004, Cardoso et al, 2005).
The trials published at the time of submission were as follows:
|
Trial ID |
Protocol title/ Publication title |
Publication citation |
|---|---|---|
|
Supportive evidence |
||
|
Bakheit et al (Study 016) |
A randomised, double-blind, placebo-controlled, dose regimen study to compare the efficacy and safety of three doses of Botulinum toxin type A (Dysport) with placebo in upper limb spasticity after stroke. |
Stroke 2000; 31:2402-6 |
|
Bakheit et al, (Study 049) |
A randomized, double-blind, placebo-controlled study of the efficacy and safety of botulinum toxin type A in upper limb spasticity in patients with stroke |
European Journal of Neurology 2001, 8: 559-565 |
|
Supplementary Published reports |
||
|
Bhakta et al, 2000 |
Impact of botulinum toxin type A on disability and carer burden due to arm spasticity after stroke: a randomized double blind placebo controlled trial |
J Neurol Neurosurg Psychiatry 2000 69: 217-221 |
|
Bakheit et al, 2004 |
The beneficial antispasticity effect of botulinum toxin type A is maintained after repeated treatment cycles |
J Neurol Neurosurg Psychiatry 2004, 75:1558-61 |
|
Meta-analyses of direct randomised trials |
||
|
Francis et al, 2004 |
Does reducing spasticity translate into functional benefit? An exploratory meta-analysis. |
J Neurol Neurosurg Psychiatry 2004, 75:1547-51 |
|
Cardoso et al, 2005 |
Botulinum toxin A for the treatment of upper limb spasticity after stroke: A meta-analysis. |
Arq. Neuropsiquiatr 2005. 63(1): 30-33 |
8. Results of Trials
The primary outcome of the key trial (097) was the changes in the Assessment of Quality
of Life (AQoL) scores from week 0 to week 20. Relevant secondary outcomes included
changes in the Modified Ashworth Scale (MAS), the Goal Attainment Scaling score (GAS)
and the Global Assessment of Benefit (GAB) score.
The difference in the mean AQoL between the botulinum toxin type A and placebo groups
from week 0 to week 20 was not statistically significant.
A statistically significantly larger number of patients in the botulinum toxin type
A group had a decrease in MAS of at least 1 point in at least one joint at week 20
than in the placebo group. The mean change in GAS score at week 20 in the botulinum
group was 5.28 higher than in the placebo group and the number of patients seeing
positive benefit of treatment on the GAB score with botulinum treatment was higher
compared with placebo treatment.
There was a wide variation in baseline AQoL scores, as illustrated in the figure below.
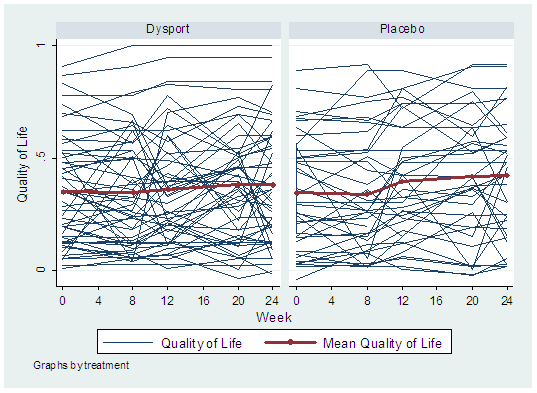
Trajectory plot of Quality of Life Scores at Weeks 0, 8, 12, 20, and 24, according
to treatment group. The mean value at each time point is also displayed.
The submission presented new toxicity data. The key results are summarised below.
Deaths, Severe Adverse Effects (SAEs) and Discontinuations in Study 097
|
Deaths |
SAEs (not treatment related) |
Discontinuations |
||
|---|---|---|---|---|
|
Dysport |
Placebo |
Dysport |
Placebo |
|
|
0 |
5/54 |
6/42 |
1/54 |
2/42 |
The PBAC noted that the submission did not provide further details on the serious
adverse effects but stated that the results from Study 097 and other supportive studies
gave no cause of concern regarding the safety of clostridium botulinum toxin type
A-haemagglutinin complex injection.
For PBAC’s comments on these results, see Recommendation and Reasons.
9. Clinical Claim
The submission claimed that clostridium botulinum toxin type A-haemagglutinin complex
injection is more effective and associated with more toxicity compared to standard
management treatment.
For further PBAC’s view of this claim, see Recommendation and Reasons
10. Economic Analysis
The submission presented an updated preliminary trial-based economic evaluation. The
submission presented the mean change in MAS score of >1 in at least one joint as the
outcome measure for economic evaluation. A cost effective analysis based on MAS change
was presented instead of a cost utility analysis.
The trial-based incremental cost per extra responder was estimated by the submission
to be less than $15,000. A responder had a change in MAS score of >1.
A modelled economic evaluation was not provided for the economic evaluation.
11. Estimated PBS Usage and Financial Implications
The likely number of patients per year was estimated by the submission to be less
than 10,000 in year 5.
The financial cost per year to the PBS was estimated by the submission to be less
than $10 million in year 5.
The submission proposed a Quality Use of Medicines (QUM) program where the sponsor
would train neurologists and rehabilitation specialists on dose selection and injection
technique in the post-stroke spasticity setting.
The submission also proposed a risk sharing agreement.
12. Recommendation and Reasons
The PBAC recommended an extension to the Section 100 botulinum toxin program to include
the treatment of moderate to severe spasticity of the upper limb in adults following
a stroke, as second line therapy when standard management has failed or as an adjunct
to physical therapy, on a the basis of an acceptable cost-effectiveness ratio compared
with standard management (placebo).
The PBAC considered the restriction proposed in the sponsor’s pre-subcommittee response
to be appropriate but should specify that the maximum number of treatments authorised
be 4 per upper limb and per lifetime. The PBAC noted that Medicare Australia would
need to be consulted about the wording of the restriction because prescriptions for
botulinum are not authorised in the usual manner. Also the prescribers authorised
to initiate treatment with botulinum were not specified, but should include neurologists
and rehabilitation specialists.
The PBAC noted that the objective of the pivotal study 097 on which the submission
was based was to show that the change in spasticity was functionally relevant to the
patient. The trial had quality of life measured by the AQoL multi-attribute instrument
as its primary outcome, but there was no statistical difference between placebo and
botulinum. On the other hand, disease specific functional measures were included in
study 097 and they were positive for botulinum. The PBAC noted that the results of
study 097 could not be used to extrapolate to a cost utility analysis because the
study did not show an improvement in the AQoL.
The sponsor at its hearing stated that there was an inherent problem in applying global
measures like AQoL to evaluate outcomes from a focal intervention, and emphasised
that the AQoL was an unsuitable instrument based on whole body outcomes and was not
validated for focal intervention affecting upper limb only and had a large inter-
and intra- patient variation.
The PBAC noted that botulinum treatment resulted in a larger number of patients with
a decrease in MAS of at least 1 point in at least one joint at week 20 and agreed
that this change was significant. The PBAC considered that the relevance of the MAS
improvement was supported by the Goal Attainment Scaling (GAS) and the Global Assessment
of Benefit (GAB). It was noted that the mean change in GAS score at week 20 in the
botulinum group was 5.28 higher than in the placebo group and that the number of patients
seeing positive benefit of treatment on the GAB score with botulinum treatment was
higher compared with placebo treatment.
The PBAC agreed that both GAS and GAB were appropriate outcomes to measure improvement
in upper limb. The PBAC considered that the results indicated that apart from the
AQoL all other outcomes measured in the trial favoured botulinum.
The PBAC noted the information reported in the patient impact statements on what it
is like to live with spasticity of the upper limb/s following stroke.
The PBAC considered that although there was uncertainty in the size or sustainability
of the functional outcome and the impact of this on quality of life and subsequently
the cost-effectiveness that this could be mitigated by the risk-sharing agreement.
Recommendation
Add the following to the restriction under the BOTULINUM TOXIN PROGRAM:
Restriction: Treatment of moderate to severe spasticity [defined as MAS greater than
or equal to 3 using modified Ashworth scale] of the upper limb in adults following
a stroke, as second line therapy
when standard management has failed (eg physiotherapy and/or oral spasticity agents)
or as an adjunct to physical therapy.
Maximum number of treatments to be authorised is 4 per upper limb per lifetime.
Treatment should not be initiated until 3 to 6 months post-stroke in patients who
do not have established severe contracture. Treatment should be discontinued if patient
does not respond (decrease of
MAS > 1 in at least one joint) after two treatments.
Contra-indications to treatment include established severe contracture, known sensitivity
to botulinum toxin
Note: Arrangements to prescribe this item should be made by medical practitioners
with Medicare Australia, contact telephone number 1800 700 270
Pack size: 1
13. Context for Decision
The PBAC helps decide whether and, if so, how medicines should be subsidised in Australia. It considers submissions in this context. A PBAC decision not to recommend listing or not to recommend changing a listing does not represent a final PBAC view about the merits of the medicine. A company can resubmit to the PBAC or seek independent review of the PBAC decision.
14. Sponsor’s Comment
Ipsen Pty Ltd welcomes the PBAC recommendation to include Dysport (clostridium botulinum toxin type A) on the PBS for the treatment of adults with spasticity of the upper limb following a stroke.




