ALENDRONATE SODIUM, tablet equivalent to 70 mg alendronic acid, Fosamax Once Weekly®, ALENDRONATE SODIUM with COLECALCIFEROL tablet equivalent to 70 mg alendronic acid with 70 micrograms colecalciferol, Fosamax Plus®, ALENDRONATE SODIUM with COLECALCIFEROL tablet equivalent to 70 mg alendronic acid with 140micrograms colecalciferol, Fosamax Plus 70 mg/140 mcg®, ALENDRONATE SODIUM with COLECALCIFEROL and CALCIUM CARBONATE, pack containing 4 tablets containing the equivalent of 70 mg alendronic acid with 140micrograms colecalciferol and 48 tablets calcium carbonate 1.25 g (equivalent to 500 mg calcium), Fosamax Plus D-Cal® - July 2010
Page last updated: 19 October 2010
Public Summary Document
Product: ALENDRONATE SODIUM, tablet equivalent to
70 mg alendronic acid, Fosamax Once Weekly®, ALENDRONATE SODIUM
with COLECALCIFEROL tablet equivalent to 70 mg alendronic acid with
70 micrograms colecalciferol, Fosamax Plus®, ALENDRONATE SODIUM
with COLECALCIFEROL tablet equivalent to 70 mg alendronic acid with
140micrograms colecalciferol, Fosamax Plus 70 mg/140 mcg®,
ALENDRONATE SODIUM with COLECALCIFEROL and CALCIUM CARBONATE, pack
containing 4 tablets containing the equivalent of 70 mg alendronic
acid with 140micrograms colecalciferol and 48 tablets calcium
carbonate 1.25 g (equivalent to 500 mg calcium), Fosamax Plus
D-Cal®
Sponsor: Merck Sharp & Dohme (Australia) Pty
Limited
Date of PBAC Consideration: July 2010
1. Purpose of Application
The submission requested an extension to the current Authority
Required (STREAMLINED) listing to include the treatment of
corticosteroid-induced osteoporosis (CIO) in patients on long term
(≥ 3 months) high dose (≥ 7.5 mg per day prednisolone or
equivalent) corticosteroid therapy and a bone mineral density (BMD)
T-score ≤ -1.5.
2. Background
This drug had not previously been considered by the PBAC for this
indication.
3. Registration Status
Alendronate 70 mg tablets were TGA registered as of 9 February
2001, for the treatment of osteoporosis. Osteoporosis must be
confirmed by the finding of low bone mass of at least 2 standard
deviations below the gender specific mean for young adults or by
the presence of osteoporotic fracture.
Alendronate sodium 70 mg with colecalciferol 70 micrograms and
alendronate sodium 70 mg with colecalciferol 140 micrograms were
TGA registered as of 8 March 2006 and 14 May 2008 respectively, for
the treatment of osteoporosis in select patients where vitamin D
supplementation is recommended.
Alendronate sodium 70 mg with colecalciferol 140 micrograms and
calcium carbonate 1.5 g (equivalent to 500mg elemental calcium)
composite pack was TGA registered on 25 March 2010 for the
treatment of osteoporosis in select patients where vitamin D and
calcium supplementation is recommended. Prior to treatment,
osteoporosis must be confirmed by the finding of low bone mass of
at least 2 standard deviations below the gender specific mean for
young adults or by the presence of osteoporotic fracture.
4. Listing Requested and PBAC’s View
Authority required (STREAMLINED)
Treatment as the sole PBS-subsidised anti-resorptive agent for
corticosteroid-induced osteoporosis in a patient currently on
long-term (at least 3 months), high-dose (at least 7.5 mg per day
prednisolone or equivalent) corticosteroid therapy with a Bone
Mineral Density (BMD) T-score of -1.5 or less.
The duration and dose of corticosteroid therapy together with the
date, site (femoral neck or lumbar spine) and score of the
qualifying BMD measurement must be documented in the
patient’s medical records when treatment is initiated.
For PBAC’s view, see Recommendation and
Reasons.
5. Clinical Place for the Proposed Therapy
Corticosteroids are widely used in a variety of chronic
non-infectious inflammatory diseases because of their
immunosuppressant and anti-inflammatory properties. However,
patients receiving high doses of corticosteroids are at increased
risk of significant bone loss and fractures. The two most serious
adverse events reported are osteoporosis and related
fractures.
Alendronate would provide an alternative oral bisphosphonate
treatment for corticosteroid-induced osteoporosis in patients on
long-term corticosteroid therapy, who are at risk of
fracture.
6. Comparator
The submission nominated risedronate as the comparator. The PBAC
considered this was appropriate.
7. Clinical Trials
The submission presented an indirect analysis comparing alendronate with risedronate
using placebo as the common comparator. For this comparison, the submission presented
four randomised controlled trials comparing alendronate with placebo and two direct
randomised comparative trials and meta-analysis comparing risedronate and placebo
in patients with glucocorticoid-induced osteoporosis.
The trials published at the time of submission are listed below:
| Trial ID/First author | Protocol title/Publication title | Publication citation |
|---|---|---|
| Alendronate Studies | ||
| Saag et al | Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. | N Engl J Med 1998; 339:292-9 |
| Adachi et al | Two-Year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids. | Arthritis and Rheumatism 2001; 44(1): 202-211 |
| De Nijs et al | Alendronate or alfacalcidol in glucocorticoid-induced osteoporosis. | N Engl J Med 2006; 355: 675-84 |
| Stoch et al | Once-Weekly oral alendronate 70mg in patients with glucocorticoid-induced bone loss: a 12-month randomised, placebo-controlled clinical trial. | J Rheumatol 2009; 36: 1705 -14. |
| Risedronate Studies | ||
| RCT 009893 Reid D.M. | Efficacy and safety of daily risedronate in the treatment of corticosteroid-induced osteoporosis in men and women: A randomized trial | Journal of Bone and Mineral Research 2000; 15(6): 1006-1013 |
| RCP 009993 Cohen S | Risedronate therapy prevents corticosteroid-induced bone loss: A twelve-month, multicentre, randomized, double-blind, placebo-controlled, parallel-group study. | Arthritis & Rheumatism 1999; 42(11): 2309-2318 |
| Wallach 2000 (meta-analyses of direct randomised trials) | Effects of risedronate treatment on bone density and vertebral fracture in patients on corticosteroid therapy. | Calcified Tissue International 2000; 67: 277-285 |
| Kanis 2007 (systematic review) | Glucocorticoid-induced osteoporosis: a systematic review and cost-utility analysis. (Includes all trials listed above). | Health Technol Assess 2007: 11(7) |
8. Results of Trials
The efficacy of alendronate and risedronate were indirectly compared and presented
as a bone mineral density analysis and fracture risk analysis.
Bone Mineral Density (BMD) Analysis
The indirect comparison of baseline percentage changes in BMD at the femoral neck
showed that risedronate was associated with a slightly better increase in BMD compared
with alendronate although this was not statistically significant. The submission stated
that this difference was reduced if the Adachi paper, the extension study to Saag
(1998), was included which may indicate that the duration of alendronate therapy is
important. The difference may also be due to the large placebo effect seen in the
Stoch paper.
Indirect comparison of alendronate vs. risedronate via placebo as common comparator:
WMD in percentage change in BMD at the femoral neck
| Study ID and active dose group(s) of placebo controlled trials included in the indirect comparison of alendronate vs. risedronate | Indirect estimate of effect: | |||||
|---|---|---|---|---|---|---|
| alendronate vs. placebo | alendronate dose group | risedronate vs. placebo | risedronate dose group | indirect WMD | (indirect 95% CI) | p-value |
| Population: All | ||||||
| Saag 1998 | 10mg 10mg 70mg weekly | Cohen 1999 | 5 mg 5mg | -1.04 | (-3.44,1.35) | 0.393 |
| de Nijs 2006 | Reid 2000 | |||||
| Stoch 2009 | ||||||
| Adachi 2001 (Saag cont.) | 10mg 10mg 70mg weekly | Cohen 1999 | 5 mg 5mg | -0.58 | -3.51, 2.34 | 0.697 |
| de Nijs 2006 | Reid 2000 | |||||
| Stoch 2009 | ||||||
WMD, weighted mean difference
A negative WMD indicates difference in favour of risedronate
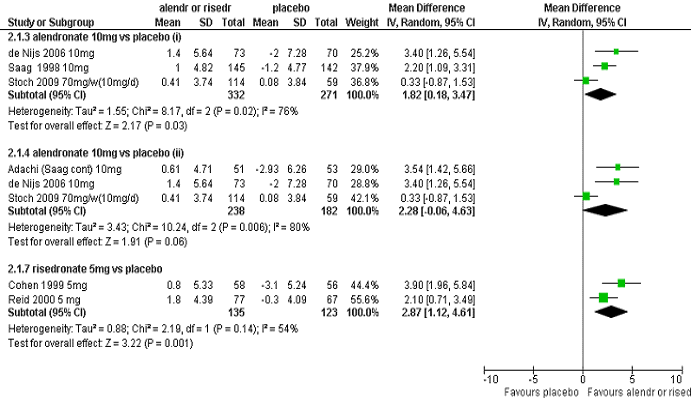
There is large statistical heterogeneity in changes in femoral neck BMD for both the
pooled analysis of the alendronate studies (I2=76%) and the risedronate studies (I2=54%), as shown in the following figure.
Forest plots showing difference in percentage change in BMD at the femoral neck (direct comparison of active therapy relative to placebo) for pooling of studies used in the indirect meta-analyses of alendronate versus risedronate via placebo – analysis for total patient population
The indirect comparison of baseline percentage changes in BMD at the lumbar spine
also showed no significant difference between alendronate and risedronate.
Indirect comparison of alendronate vs. risedronate via placebo as common comparator:
WMD in percentage change in BMD at the lumbar spine
| Study ID and active dose group(s) of placebo controlled trials included in the indirect comparison of alendronate vs. risedronate | Indirect estimate of effect: | |||||
|---|---|---|---|---|---|---|
| alendronate vs. placebo | alendronate dose group | risedronate vs. placebo | risedronate dose group | indirect WMD | (indirect 95% CI) | p-value |
| Population: All | ||||||
| Saag 1998 | 10mg 10mg 70mg weekly | Cohen 1999 | 5 mg | 0.42 | (-0.70, 1.53) | 0.464 |
| de Nijs 2006 | Reid 2000 | |||||
| Stoch 2009 | ||||||
| Adachi 2001 (Saag cont.) | 10mg 10mg | Cohen 1999 | 5 mg 5mg | 0.75 | (-0.56, 2.07) | 0.262 |
| de Nijs 2006 | Reid 2000 | |||||
WMD, weighted mean difference
A positive WMD indicates difference in favour of alendronate
Fracture Risk Analysis
The submission stated that only the overall non-vertebral and morphometric vertebral
fractures were compared as the incidence of fractures in all studies was low which
made it impossible to perform sub-group analyses.
Of the two risedronate studies, only Cohen (1999) reported non-vertebral fracture
data. The data from Cohen included all non-vertebral fractures in the 2.5 mg and 5.0
mg treatment groups. In non-vertebral fractures, the indirect comparison showed there
was a non-significant reduction in fractures in favour of risedronate when only the
Saag and de Nijs studies are included, although when the Adachi study was included
there was a non-significant reduction in non-vertebral fractures in favour of alendronate.
Indirect comparison of alendronate vs. risedronate via placebo as common comparator:
Risk ratio of non vertebral fracture
| Study ID and active dose group(s) of placebo controlled trials included in the indirect comparison of alendronate vs. risedronate | Indirect estimate of effect: | |||||
|---|---|---|---|---|---|---|
| alendronate vs. placebo | alendronate dose group | risedronate vs. placebo | r isedronate dose group | i ndirect risk ratio | (indirect 95% CI) | p-value |
| Population: All | ||||||
| Saag 1998 | 5/10 mg or 10 mg | Cohen 1999 | 2.5/5 mg | 1.21 | (0.28, 5.25) | 0.799 |
| de Nijs 2006 | ||||||
| Adachi 2001 (Saag cont.) | 5/10 mg or 10 mg | Cohen 1999 | 2.5/5 mg | 0.76 | (0.17,3.47) | 0.725 |
| de Nijs 2006 | ||||||
Indirect risk ratio less than 1 indicates result in favour of alendronate
In morphometric vertebral fractures, the indirect comparison showed no significant
difference between alendronate and risedronate. However, with the inclusion of the
Adachi study, there was an overall reduction in morphometric vertebral fractures for
alendronate that reached statistical significance. When both the Cohen and Reid studies
were combined, the reduction in morphometric vertebral fractures also reached statistical
significance vs. placebo.
Indirect comparison of alendronate vs. risedronate via placebo as common comparator:
Risk ratio of (morphometric) vertebral fracture
| Study ID and active dose group(s) of placebo controlled trials included in the indirect comparison of alendronate vs. risedronate | Indirect estimate of effect: | |||||
|---|---|---|---|---|---|---|
| alendronate vs. placebo | alendronate dose group | risedronate vs. placebo | risedronate dose group | indirect risk ratio | (indirect 95% CI) | p-value |
| Population: All | ||||||
| Saag 1998 | 5/10 mg or 10mg | Cohen 1999 | 2.5/5 mg | 1.29 | (0.43, 3.93) | 0.649 |
| de Nijs 2006 | Reid 2000 | |||||
| Adachi 2001 (Saag cont.) | 5/10 mg or 10mg | Cohen 1999 | 2.5/5 mg | 0.70 | (0.19, 2.68) | 0.607 |
| de Nijs 2006 | Reid 2000 | |||||
Indirect risk ratio less than 1 indicates result in favour of alendronate
9. Clinical Claim
The submission claimed that alendronate is non-inferior to
risedronate in the treatment or prevention of
glucocorticoid-induced osteoporosis as there is no significant
difference between alendronate and risedronate in BMD in the lumbar
spine and femoral neck and for non-vertebral and morphometric
vertebral fractures. The PBAC accepted this claim.
For PBAC’s view, see Recommendation and
Reasons.
10. Economic Analysis
As this was a minor submission, no economic analysis was
presented.
11. Estimated PBS Usage and Financial Implications
The submission stated that this listing was not expected to grow
the market and that alendronate uptake would be mainly from
patients switching from risedronate. The sponsor stated that it
would be unlikely for patients to switch from zoledronic acid once
yearly IV therapy to alendronate once weekly oral therapy,
therefore zoledronic acid had not been included in modelling uptake
estimates.
The submission estimated financial savings per year to the
Government of less than $10 million in Year 5 of listing due to the
lower dispensed price for maximum quantity (DPMQ) of alendronate
compared to risedronate.
For PBAC’s view, see Recommendation and
Reasons.
12. Recommendation and Reasons
The PBAC recommended extending the listing of alendronate and its
combinations with calcium and/ or colecalciferol to include an
Authority Required (Streamlined) listing for the treatment of
corticosteroid-induced osteoporosis in patients currently on
long-term (at least 3 months), high-dose (at least 7.5 mg per day
prednisolone or equivalent) corticosteroid therapy with a Bone
Mineral Density (BMD) T-score of -1.5 or less on a cost
minimisation basis with risedronate sodium at the price proposed in
the submission. The equi-effective doses are 70 mg alendronic acid
weekly and 35 mg risedronate sodium weekly.
The PBAC considered, based on the totality of the evidence
presented, that alendronate is of non-inferior efficacy and safety
to risedronate in the treatment of corticosteroid-induced
osteoporosis. The PBAC noted that the submission presented an
indirect analysis comparing alendronate with risedronate using
placebo as the common comparator. For this comparison, the
submission presented four randomised controlled trials comparing
alendronate with placebo, and two direct randomised comparative
trials and a meta-analysis comparing risedronate and placebo in
patients with glucocorticoid-induced osteoporosis. The PBAC noted
that although some of the alendronate studies used 5 mg or 10 mg
once daily doses, Stoch 2009 used the 70 mg once weekly dose. The
PBAC also noted that it had previously recommended the listing of
alendronate 70 mg tablets taken once weekly on a cost minimisation
basis with the 10 mg tablet once daily for the treatment of
osteoporosis and accepted that the two dosage regimens provided
similar safety and efficacy.
The PBAC agreed that the listing of alendronate would not be
expected to grow the market for corticosteroid-induced osteoporosis
and considered that alendronate uptake is likely to be mainly from
patients switching from PBS subsidised risedronate, and hence that
the listing of alendronate for corticosteroid-induced osteoporosis
would not be expected to result in any increased financial cost to
the Government. The PBAC also considered the submission’s
claim that it is unlikely that patients would switch from treatment
with zoledronic acid once yearly I.V. to alendronate once weekly
orally, as reasonable.
Recommendation:
ALENDRONATE SODIUM, tablet equivalent to 70 mg alendronic acid,
Fosamax Once Weekly®, ALENDRONATE SODIUM with
COLECALCIFEROL, tablet equivalent to 70 mg alendronic acid with 70
micrograms colecalciferol, Fosamax Plus®,
ALENDRONATE SODIUM with COLECALCIFEROL, tablet equivalent to 70 mg
alendronic acid with 140 micrograms colecalciferol, Fosamax Plus 70
mg/140 mcg®, ALENDRONATE SODIUM with COLECALCIFEROL
and CALCIUM CARBONATE, pack containing 4 tablets containing the
equivalent of 70 mg alendronic acid with 140 micrograms
colecalciferol and 48 tablets calcium carbonate 1.25 g (equivalent
to 500mg calcium)
Extend the current restriction to include:
Restriction: Authority required (STREAMLINED)
Treatment as the sole PBS-subsidised anti-resorptive agent for corticosteroid-induced osteoporosis in a patient currently on long-term (at least 3 months), high-dose (at least 7.5 mg per day prednisolone or equivalent) corticosteroid therapy with a Bone Mineral Density (BMD) T-score of -1.5 or less.
The duration and dose of corticosteroid therapy together with the date, site (femoral neck or lumbar spine) and score of the qualifying BMD measurement must be documented in the patient’s medical records when treatment is initiated.
Maximum quantity:
4 (70 mg, 70 mg/70 mcg and 70 mg/140 mcg)
‡1 (70 mg/140 mcg with 1.25 g calcium)
Repeats: 5
13. Context for Decision
The PBAC helps decide whether and, if so, how medicines should be
subsidised in Australia. It considers submissions in this context.
A PBAC decision not to recommend listing or not to recommend
changing a listing does not represent a final PBAC view about the
merits of the medicine. A company can resubmit to the PBAC or seek
independent review of the PBAC decision.
14. Sponsor’s Comment
The sponsor welcomes the PBAC's decision to make alendronate
available to patients with corticosteroid-induced osteoporosis.




