Miglustat, capsule, 100 mg, Zavesca® - November 2011
Page last updated: 02 March 2012
PDF printable version of Miglustat, capsule, 100 mg, Zavesca® (PDF 87 KB)
Public Summary Document
Product: Miglustat, capsule, 100 mg,
Zavesca®
Sponsor: Actelion Pharmaceuticals Australia Pty
Ltd
Date of PBAC Consideration: November 2011
1. Purpose of Application
The re-submission requested inclusion in the Life Saving Drugs
Program (LSDP) for the treatment of progressive neurological
manifestations in adults and paediatric patients with Niemann-Pick
type C (NP-C) disease.
Through the LSDP, the Australian Government provides subsidised
access, for eligible patients, to expensive and potentially life
saving drugs for very rare life-threatening conditions. Before a
drug is made available on the LSDP, it must generally be accepted
by the Pharmaceutical Benefits Advisory Committee (PBAC) as
clinically necessary and effective, but not recommended for
inclusion on the Pharmaceutical Benefits Scheme due to unacceptable
cost-effectiveness.
Subsidised access through the LSDP is granted in accordance with
specified eligibility criteria and subject to certain conditions:
Life Saving Drugs Program Criteria and Conditions (PDF 17 KB)
2. Background
At the July 2010 meeting, the PBAC rejected an application to
include miglustat on the PBS for the treatment of NP-C disease on
the basis of uncertain clinical efficacy and a very high and
uncertain cost effectiveness ratio. The PBAC considered that
miglustat for the treatment of NP-C disease did not meet the
criteria for the LSDP, and hence was not suitable for consideration
of inclusion in the LSDP.
See July 2010 Public Summary Document for full
details.
3. Registration Status
Miglustat was TGA registered on 3 February 2010 for the treatment
of progressive neurological manifestations in adult and paediatric
patients with NP- C disease.
4. Listing Requested and PBAC’s View
Authority required
Treatment of progressive neurological manifestations in adults and
paediatric patients with Niemann-Pick disease Type C disease.
For PBAC’s view, see Recommendation and
Reasons.
5. Clinical Place for the Proposed Therapy
NP-C disease is a very rare inherited disorder that is progressive,
debilitating, degenerative and ultimately fatal, affecting the
liver, lungs, bone marrow and brain. The neurological
manifestations are caused by the accumulation of lipids primarily
in the brain.
Treatment for NP-C disease is currently palliative only and depends
on the needs of the individual, the symptoms and the clinical
manifestations.
The submission proposed that miglustat will provide a pharmacologic
intervention that may interrupt disease progression.
6. Comparator
The submission nominated standard medical management comprising of
palliative care.
At the July 2010 meeting, the PBAC considered that placebo plus
standard medical management (standard care) was the appropriate
comparator, noting that miglustat would be used in addition to,
rather than replacing, standard care.
7. Clinical Trials
The previously submitted trials were:
- Randomised controlled trial OGT918-007a and its extension study (OGT918-007a(ext));
- Paediatric sub-study of the above trial (OGT918-007p) and its extension study (OGT918-007p(ext));
- Stage I survey; and
- Two case-series and 19 case reports.
The new data presented in the submission were from:
- Three videofluoroscopy swallowing studies (VFSSs) of up to six patients (Australian, Italian and Taiwanese); and
- Other patient case series data: UK data, Spanish and Portuguese data, and Italian data;
- Survival analysis of patients treated with miglustat compared with an untreated cohort compiled from several patient sources.
Details of the studies published at the time of submission are shown in the table below:
| Trial ID/First Author | Protocol title/ Publication title | Publication citation |
|---|---|---|
| RCT data | ||
| Trial OGT 918-007a | ||
| Patterson MC et al 2007 | Miglustat for treatment of Niemann-Pick C disease: a randomised controlled study. | Lancet Neurology 2007; 6(9): 765-772. |
| RCT extension data | ||
| Study OGT 918-007p | ||
| Patterson MC et al 2007 | Miglustat for treatment of Niemann-Pick C disease: a randomised controlled study. | Lancet Neurology 2007; 6(9): 765-772. |
| Study OGT 918-007a (ext) | ||
| Wraith JE et al 2009 | Miglustat in adult and juvenile patients with Niemann-Pick disease type C: Long-term data from a clinical trial. | Molecular Genetics and Metabolism, epub 30 December 2009. |
| Study OGT 918-007p (ext) | ||
| Patterson MC et al 2010 | Long-term miglustat therapy in children with Niemann-pick disease Type C. | Journal of Child Neurology 2010; 25: 300-305. |
| Videofluoroscopy data | ||
| Italian case studies Feracotta S et al 2011 | The videofluoroscopic swallowing study shows a sustained improvement of dysphagia in children with Niemann-Pick disease type C after therapy with miglustat . | American Journal of Medical Genetics, Part A 2011; 155: 540-547. |
| Brushini D et al 2008 | Efficacy of Miglustat on Dysphagia in Four Niemann Pick Patients . | Journal of Inherited Metabolic Disease 2008; 31 (Sup 1):123. |
| Taiwanese case studies Chien YH et al 2007 | Treatment of Niemann-Pick disease type C in two children with miglustat: Initial responses and maintenance of effects over 1 year. | JIMD Short Reports #063 (2007) DOI 10.1007/S 10545-007-0630-Y. |
| Other patient data | ||
| UK data Jacklin E et al 2010 | Review of 11 patients with NPC1 treated with miglustat. | Molecular Genetics and Metabolism. 2010; 99: S22. |
| Spanish and Portuguese data Pineda M et al 2010 | Clinical experience with miglustat therapy in paediatric patients with Niemann–Pick disease type C: A case series . | Molecular Metabolism. 2010; 99: 358-366. |
| Italian data Fecarotta S et al 2009 | Efficacy of miglustat on the neurological involvement in Italian patients with Niemann-Pick disease type C. | Molecular Genetics and Metabolism 2009; 98: 70 (Abstract). |
| Pineda M et al 2009 | Miglustat in patients with Niemann-Pick disease Type C (NP-C): a multicenter observational retrospective cohort study. | Molecular Genetics and Metabolism 2009; 98 (3):243-249. |
| Sedel F 2010 | Changing the disease course: Disease modifying treatment for Niemann-Pick type C. | European Neuropsycho-pharmacology. 2010; 20(Sup 3): S639. |
NP-C = Niemann-Pick disease Type C; RCT = randomised
controlled trial
8. Results of Trials
The instruments used in the three videofluoroscopy case studies included the Dysphagia
Severity Scale, Han Functional Dysphagia Scale, Penetration/Aspiration Scale, and
Melbourne Health VFSS Rating Scale.
Overall, the results from the VFSSs showed a high degree of variation across subjects.
The re-submission did not provide information regarding whether the swallowed material
used in the assessment of swallowing function was consistent across studies or across
patients. Due to a lack of pre-treatment assessment for most of the patients (and/or
a control group), it could not be determined whether the observed changes in swallowing
function during the treatment period were due to natural disease progression or resulted
from the use of miglustat. Given that the rate of progression of neurological symptoms
in NP-C, including swallowing function, can vary over the course of the disease, and
there may also be temporary periods of spontaneous improvement, the lack of baseline
data and/or a control group severely hindered interpretation of the swallowing study
results.
Additional patient data:
The data are based on a small number of patients, with large intra- and inter-individual
variability in rates of disease progression. The utility of the results was further
limited by the potential for observer bias.
Longitudinal analysis of survival data:
A significantly lower risk of mortality was reported in the miglustat cohort than
in the untreated cohort (hazard ratio: 0.16 [0.063, 0.391]), based on 10 years of
patient data after the onset of neurological symptoms for both groups.
The Kaplan-Meier survival curves for the treated and untreated cohort of patients
with NP-C are shown below:
Kaplan-Meier survival curves for treated and untreated cohort of patients with NP-C
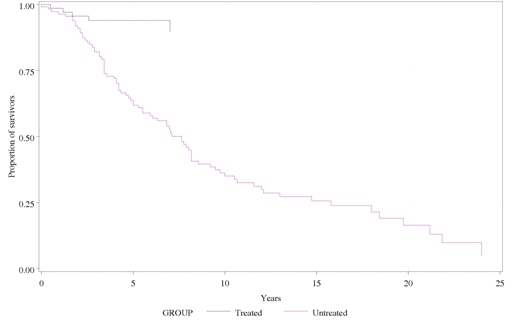
The survival curves of the two cohorts diverge within the first 3 years after the
onset of neurological involvement.
The Pre-Sub-Committee Response (PSCR) stated that the data used in the survival analysis
are real world data of patients and that these patients are reflective of the likely
patient population to receive miglustat if funded on the LSDP.
The survival curves, time from miglustat treatment initiation compared with time from
symptom onset in the control arm, are shown in the figure below:
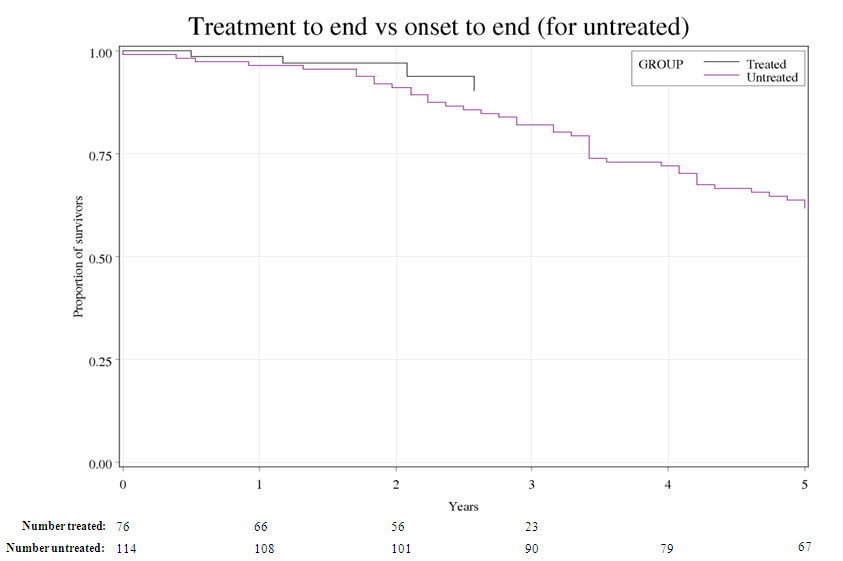
For PBAC’s view of these results, see Recommendation and Reasons.
No new toxicity data from clinical studies were presented in the re-submission.
9. Clinical Claim
The re-submission described miglustat as superior to placebo in
terms of effectiveness in patients with NP-C.
Based on the data presented in the resubmission, the PBAC did not
accept the clinical claim that miglustat is superior to placebo in
terms of effectiveness. The PBAC also reaffirmed its previous
conclusion from July 2010 that miglustat is not as safe as
placebo.
10. Economic Analysis
An updated modelled economic evaluation was not presented.
At the July 2010 PBAC meeting, the PBAC accepted that miglustat is
unlikely to be cost-effective.
11. Estimated PBS Usage and Financial Implications
The submission estimated the likely number of patients/year to be
less than 10,000 in the first 5 years.
The net financial cost to the PBS was estimated by the submission
to be less than $10 million in Year 5.
The estimates were uncertain as the prevalence and incidence of
NP-C disease in the Australian population is uncertain, and
therefore so are the number of patients likely to be treated.
12. Recommendation and Reasons
The PBAC noted that NP-C disease is highly heterogeneous in nature
and characterised by a widely varying life expectancy and wide
variability in age of onset and clinical presentations. The PBAC
acknowledged the scarceness of clinical data for the use of
miglustat in the treatment of patients with NP-C disease given the
rarity of NP-C disease.
However, based on the data presented in the resubmission, the PBAC
did not accept the clinical claim that miglustat is superior to
placebo in terms of effectiveness. The PBAC also reaffirmed its
previous conclusion that miglustat is not as safe as placebo.
The PBAC noted the new clinical data presented in the
re-submission, which comprised three videofluoroscopy swallowing
studies (VFSSs) of up to six NP-C patients (Australian, Italian and
Taiwanese), other NP-C patient case series data (UK data, Spanish
and Portuguese data, and Italian data), and a survival analysis of
NP-C patients treated with miglustat compared with an untreated
N-PC cohort sourced from elsewhere.
Regarding the VFSSs, the PBAC considered that the evidence provided
was not sufficient to support the validity and reliability of the
four scales used in the studies. Additionally, given the lack of
blinded outcome assessment there is potential for
investigator/assessor bias in the assessment of the subjective
outcome of swallowing function.
The PBAC noted that the results of the three VFSSs did not show
consistent improvement or stabilisation across treated patients in
swallowing function/dysphagia scores and there was no information
on a control group of either untreated NP-C patients or consistent
results prior to treatment. The PBAC considered it is therefore
unknown from these results whether miglustat mitigates disease
progression as measured by dysphagia.
Regarding the NP-C patient case series data, the PBAC noted that
the composite scales used to measure NP-C disease severity in the
UK, Spanish and Portuguese patients have not been validated. The
data are based on a small number of patients, with large intra- and
inter-individual variability in rates of disease progression. The
utility of the results is further limited by the potential for
observer bias.
Regarding the longitudinal analysis of survival data presented in
the re-submission, the PBAC noted that a significantly lower risk
of mortality was reported in the miglustat cohort than in the
untreated cohort (hazard ratio: 0.16 [0.063, 0.391]), based on 10
years of patient data after the onset of neurological symptoms for
both groups. The survival data on both cohorts were pooled from a
variety of patient sources, but there was no explanation of how
these patients were selected. The comparability of the two cohorts
in terms of potential confounding factors, eg age of neurological
symptom onset, was not assessed in the re-submission. The PBAC
noted that the survival curves of the two cohorts diverged within
the first 3 years after the onset of neurological involvement.
However, the observed lower mortality in the treated cohort may
have resulted from less severe or slower natural disease
progression of NP-C in the treated patients rather than miglustat
therapy.
The PBAC noted that the Pre Sub-Committee Response re-created
survival curves looking at time from symptom onset in the control
group compared with time from treatment initiation in the miglustat
group. It states that the divergence of the survival curves from
the time of treatment initiation is again significant (test
p=0.0192). The PBAC considered that the interpretation of the
survival data remains unclear; given that patient baseline
characteristics were unmatched and that, due to more extensive
right-censoring in the miglustat cohort, the most reliable data
were at the top of the curves, before they diverge. Although the
applicant has emphasised the representativeness of patients in the
miglustat cohort to patients taking miglustat “in the real
world”, the PBAC’s concern is the comparability of the
two cohorts in terms of all aspects that might influence survival
of NP-C patients, so that the reported statistically significant
difference between the survival of the two cohorts can be
confidently attributed to miglustat or not. This concern is not
addressed either by assessing the effect of age on survival across
all treated and untreated patients, or by restricting the survival
analyses to patients who have been included in published
data.
The PBAC noted the articles in the re-submission reporting on
dysphagia and aspiration pneumonia in patients with
neurodegenerative disorders or stroke. A total of 12 cohort studies
were identified. A low to moderate degree of heterogeneity in study
results was observed across the studies (I2: 23%,
Chi2 p-value: 0.21). The overall estimate of the odds
ratio (OR) of aspiration pneumonia in patients with dysphagia is
14.12 (95% CI: [13.95, 14.38]). The PBAC considered that, although
dysphagia may increase the risk of aspiration pneumonia in patients
with stroke and neurodegenerative disorders, the applicability to
NP-C patients is less clearly established.
The PBAC noted the literature search used to identify studies
providing data on aspiration pneumonia (or pneumonia) and
mortality. The results of a meta-analysis of the identified six
cohort studies which examined the relationship between aspiration
pneumonia and mortality, using a Mantel-Haenszel fixed-effects
model indicated an increased risk of mortality was observed in
patients experiencing aspiration pneumonia, with pooled OR of 3.23
(95%: [2.46, 4.25]), which is not as strong a signal as the OR of
aspiration pneumonia in patients with dysphagia. Results showed a
high degree of consistency across studies (I2: 0%,
Chi2 p-value: 0.64). However, none of the six identified
studies were in patients with NP-C (3 in stroke patients, 1 in
patients with epilepsy, 1 in patients with Parkinson’s
disease, and 1 in a population with a variety of neurodegenerative
disorders). Therefore, the PBAC again considered that the
applicability of these results to NP-C patients is uncertain.
The PBAC noted that no evidence was presented to allow an
assessment of the benefits of early intervention with miglustat for
pediatric patients and how this may alter disease progression and
life expectancy, given that the presentation of NP-C disease is
different in children compared to adults.
The PBAC therefore rejected the re submission for the inclusion of
miglustat in the Life Saving Drugs Program (LSDP) for the treatment
of progressive neurological manifestations in adults and paediatric
patients with Niemann-Pick type C (NP-C) disease on the basis that
the clinical evidence presented failed to show that miglustat meets
eligibility criterion 4 and 5 of the LSDP.
In relation to the new clinical evidence in the re-submission to
address criterion 5 of the LSDP, the PBAC did not accept that the
several sets of case series presenting dysphagia results (including
the VFSSs) provided a convincing basis to conclude that miglustat
is clinically effective for this outcome. The PBAC also did not
accept that the comparison of survival across treated and untreated
patients enabled a conclusion of clinical effectiveness because it
could not assess the comparability of the two cohorts for other
aspects that might influence survival and so could not exclude
sources of confounding. For these reasons, together with the
extremely high cost per patient per year the PBAC re-affirmed its
previous decisions not to recommend listing on the PBS and not to
accept that miglustat meets eligibility criterion 4 of the
LSDP.
In relation to the new clinical evidence in the re-submission to
address criterion 4 of the LSDP, the PBAC did not accept the
comparison of survival for the reasons already identified in
relation to criterion 5. The PBAC also did not accept that the
chain of argument presented in the re-submission (i.e., from a
treatment effect of miglustat on dysphagia to a treatment effect of
miglustat on extending a patient’s lifespan via a reduction
in aspiration pneumonia) was sufficiently established. Based on
data in other diseases, the association between dysphagia and
aspiration pneumonia is more strongly established than between
aspiration pneumonia and death. However, the applicability of these
associations to NP-C patients is uncertain. More importantly, a
clear treatment effect of miglustat on dysphagia, the first link in
this chain of argument, was not accepted for the reasons already
identified in relation to criterion 5. Overall, the PBAC
re-affirmed its previous decision not to accept that miglustat
meets eligibility criterion 4 of the LSDP.
The PBAC expressed the view that, although the new clinical
evidence provided for its consideration was not sufficient to
change its previous conclusions, comparing survival curves across
treated and untreated cohorts which are otherwise similar in terms
of characteristics with known prognostic value would be
informative. Identifying patients who would be most likely to gain
an extension in their lifespan from treatment may also be
informative.
The PBAC also noted that, relevant to criterion B2 for the LSDP,
the cost per patient for treating NP-C disease is double that for
treating Gaucher disease under the LSDP based on the relevant
recommended doses being double in NP-C disease compared with
Gaucher disease and an identical cost per pack.
The PBAC also acknowledged and noted the consumer comments on this
item.
Recommendation:
Reject
13. Context for Decision
The PBAC helps decide whether and, if so, how medicines should be
subsidised in Australia. It considers submissions in this context.
A PBAC decision not to recommend listing or not to recommend
changing a listing does not represent a final PBAC view about the
merits of the medicine. A company can resubmit to the PBAC or seek
independent review of the PBAC decision.
14. Sponsor’s Comment
Actelion acknowledges the difficulties in meeting the LSDP criteria in a very rare condition such as Niemann-Pick Disease Type C. Actelion remains committed to working with the PBAC and LSDP to ensure that patients with Niemann-Pick Disease Type C have funded access to treatment.




