Ipilimumab, concentrate solution for I.V. infusion, 50 mg in 10 mL, 200 mg in 40 mL, Yervoy® - March 2012
PDF printable version of this page (PDF 91 KB)
Public Summary Document
Product: Ipilimumab, concentrate solution for I.V. infusion, 50 mg in 10 mL, 200 mg in 40 mL, Yervoy®
Sponsor: Bristol-Myers Squibb Australia Pty Ltd
Date of PBAC Consideration: March 2012
1. Purpose of Application
The re-submission sought a Section 100 (Highly Specialised Drugs Program) Private Hospital Authority Required and Public Hospital Authority Required (STREAMLINED) listing for the treatment of patients with unresectable stage III or stage IV malignant melanoma who have not responded to or were intolerant to prior systemic therapy for metastatic disease.
Highly Specialised Drugs are medicines for the treatment of chronic conditions, which, because of their clinical use or other special features, are restricted to supply to public and private hospitals having access to appropriate specialist facilities.
2. Background
This was the second consideration by the PBAC of an application to list ipilimumab.
At its July 2011 meeting, the PBAC rejected an application to list ipilimumab for the treatment of unresectable stage III or stage IV malignant melanoma in patients who did not respond to or were intolerant of prior systemic therapy on the basis of uncertain extent of clinical benefit, uncertain clinical place of therapy, high and uncertain cost-effectiveness ratio and uncertain financial costs.
A copy of the Public Summary Document (PSD) from the July 2011 PBAC meeting is available at the Public Summary Document for-ipilimumab - July 2011.
3. Registration Status
Ipilimumab was TGA registered on 4 July 2011 as monotherapy, for the treatment of patients with unresectable or metastatic melanoma who have failed or are intolerant to prior therapy.
4. Listing Requested and PBAC’s View
Section 100 (Highly Specialised Drugs Program)
Private Hospital Authority Required
Public Hospital Authority Required (STREAMLINED)
For the treatment of patients with unresectable stage III or stage IV malignant melanoma who have failed or are intolerant to prior therapy for metastatic disease:
- where lack of response is determined by clinically verifiable measures, and is defined as failure to achieve or sustain an objective response (partial or complete response) or stable disease;
- where intolerance to prior therapy is defined as Grade 3 or 4 toxicity that is therapy related.
Note that for patients who commence therapy with ipilimumab:
- Decisions concerning efficacy should await completion of the entire induction regimen (four doses) and should be made in conjunction with established criteria for immunological responses. However, induction may be ceased or delayed if symptomatic progressive disease or intolerable adverse events occur and if, in the opinion of the clinician, continuation of treatment poses a risk to the patient.
- Tumour responses may occur beyond the initial 12-week induction phase and evaluation for potential later responses should be undertaken regularly for the first year.
- Re-induction with 4 additional doses of ipilimumab should only be commenced in patients
whose disease has progressed following an initial objective response to therapy: o
Where response to therapy is defined as either:
(i) sustained stable disease of ? 3 months duration; or
(ii) achievement of an initial objective response (partial or complete response).
For PBAC’s view, see Recommendation and Reasons.
5. Clinical Place for the Proposed Therapy
Melanomas are malignant tumours derived from melanocytes. Advanced melanoma (unresectable stage III to stage IV or metastatic melanoma) is an aggressive and invasive disease, with a median survival of approximately 6 to 9 months.
The aim of treatment in advanced melanoma is to optimally manage each stage of disease with a view to extending overall survival. Therapies for advanced melanoma are limited and include systemic therapy (dacarbazine, fotemustine or temozolomide), palliative care/radiotherapy, palliative surgery or no treatment.
The resubmission again proposed the place in therapy for ipilimumab is as a first-in-class agent for second line treatment of advanced melanoma.
6. Comparator
The resubmission nominated dacarbazine (DTIC) and fotemustine as the main comparators. This was previously accepted by the PBAC as appropriate.
7. Clinical Trials
As previously, the submission presented Study CT-020, a phase III, randomised double-blind multicentre trial in HLA-A*0201-positive (HLA-A+-positive) subjects with a diagnosis of unresectable Stage III or Stage IV melanoma who had relapsed, failed, or were not able to tolerate at least 1 or more prior treatment regimens. Publication details have been previously reported in the July 2011 PSD.
8. Results of Trials
The re-submission presented pooled results from the ipilimumab monotherapy and ipilimumab and gp100 combination therapy arms, to support the effectiveness of ipilimumab and the claim of a plateau effect in the survival curve, in response to PBAC’s previous concerns.
The survival curves from Study CT-020 are reported below:
Survival Curves from CT-020 (ITT population)
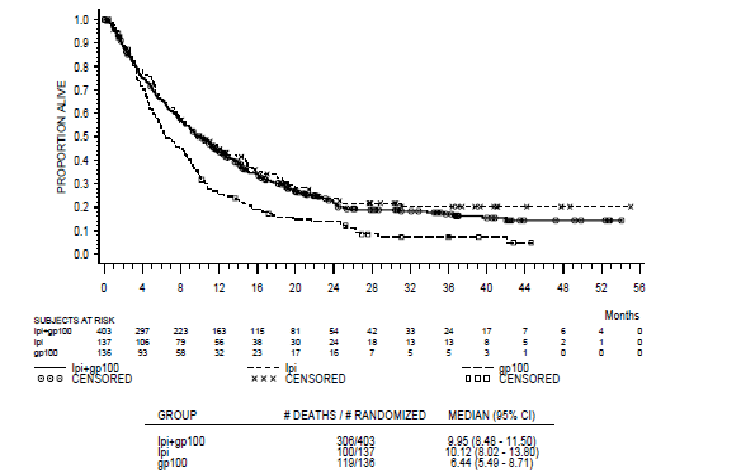
The PBAC noted that the analysis of the plateau effect in the survival curves from Study CT-020 shows 13.7% survival at 2 years in the control arm of the ipilimumab studies. However, the plateau was less convincing when the two ipilimumab containing arms are pooled. The PBAC further noted that a plateau effect is reported for melanoma as a nature of the condition. The American Joint Committee on Cancer (AJCC) reports 10-20% of Stage IV patients are alive at 10 years, however, Balch et al (Final Version of 2009 AJCC Melanoma Staging and Classification. J Clin Oncol 2009; 27:6199-6206), shows that less than 10% of Stage IV patients are alive at 10 years. Overall, the PBAC considered that there is some evidence of a plateau effect with ipilimumab treatment but the durability of this effect remains unknown.
The following table summarises the survival benefit seen in Trial CT-020.
Summary of survival data – trial based
| Survival data from CT-020 | Ipilimumab + gp100 | Ipilimumab monotherapy | gp100 monotherapy | Incremental survival (ipi vs. gp100) |
|---|---|---|---|---|
| Mean# |
N/R |
17.96 months |
10.88 months |
7.08 months |
| Median |
10.0 months |
10.1 months |
6.4 months |
3.7 months |
The PBAC noted that the observation time in the ipilimumab arms was longer than the gp100 arm, which may bias the mean overall survival results in favour of ipilimumab.
For PBAC’s view, see Recommendation and Reasons.
The resubmission presented additional exploratory analyses relating to the safety profiles of ipilimumab and dacarbazine. The additional analyses were in response to the PBAC Minutes noting that immune-response adverse events (irAEs) are higher in the ipilimumab arm. The resubmission claimed that early management of irAEs (predominantly with steroids) is capable of minimising their impact. The resubmission then claimed that experience with the irAEs of ipilimumab means they are increasingly likely to be treated early, leading to a reduction in their negative consequences, thus the results from CT-020 overstate the actual adverse event profile of ipilimumab. The PBAC agreed that this was reasonable. The resubmission then considered the AE profile of dacarbazine, which is part of the treatment pathway for BSC. The adverse event profile of fotemustine was not reconsidered in the resubmission, however, the PBAC noted that it is associated with haematological AEs. The analysis undertaken contrasted the dacarbazine monotherapy arm of a recent first-line ipilimumab study CT-024 (published as Robert et al. 2011) with the ipilimumab arm of CT-020. The PBAC considered that this is difficult to interpret due to the considerable differences in baseline characteristics of patients. Therefore, while Study CT-020 may overstate the AE profile associated with ipilimumab (and this claim is unproven), the new evidence did not provide additional information regarding the relative toxicity profiles of ipilimumab and BSC.
9. Clinical Claim
The resubmission’s claim that ipilimumab 3 mg/kg is superior in efficacy, with a different safety profile, with irAEs which are manageable and controllable, to BSC (DTIC/fotemustine) in the treatment of unresectable or metastatic melanoma patients who have received prior therapy was unchanged from the previous submission.
The PBAC noted that whilst the evidence suggests that ipilimumab is superior in terms of comparative effectiveness, ipilimumab may be considered inferior in terms of immune-related adverse events. The resubmission provided additional evidence relating to these events, identifying them as relatively manageable.
The PBAC considered that it is very difficult to quantify whether the overall safety profile of ipilimumab is or is not inferior to BSC as a direct comparison cannot be made.
10. Economic Analysis
The resubmission presented an updated modelled economic evaluation including changes to the model structure, new utility weights, and extrapolation from the end of the Kaplan-Meier curves using a within-trial hazard approach (averaged using 3 years of data). The previous model included health states for progression and no progression. The current model pooled non-progressed and progressed patients into one group (alive), reflecting the uncertainty about the appropriateness of conventional measures of progression in immunotherapeutic interventions i.e. patients who are responding to treatment may be classified as having progressed using conventional measures. The PBAC did not previously identify the economic model structure as a key uncertainty. The PBAC considered the two health state model presented in the resubmission may be an oversimplification.
Disutilities from adverse events were included in sensitivity analyses only.
The base case incremental cost per QALY gained was between $45,000 and $75,000.
The PBAC noted that the extrapolation method, and point at which extrapolation occurs, increased the ICER to between $75,000 and $105,000 per QALY gained and that sensitivity analyses show that the model is sensitive to a time horizon of 5 years and choice of utility weights.
For PBAC’s view, see Recommendation and Reasons.
11. Estimated PBS Usage and Financial Implications
The likely number of patients per year was estimated in the submission to be less than 10,000 in Year 5, at an estimated net cost per year to the PBS of between $30 and $60 million in Year 5.
For PBAC’s view, see Recommendation and Reasons.
12. Recommendation and Reasons
The PBAC considered that the place of ipilimumab in the treatment of malignant melanoma was still difficult to ascertain as the treatment algorithm is still evolving and the impact of the BRAF inhibitors (which are in clinical trials use in Australia) upon this algorithm is also unknown at this stage. The PBAC again acknowledged the high clinical need for a drug to treat malignant melanoma. However, the PBAC was concerned that the submission’s proposed clinical treatment algorithm, in which ipilimumab is available after failure or intolerance to prior systemic therapy, would inappropriately expose patients to first-line chemotherapy with dacarbazine or fotemustine for the sole purpose of gaining access to second-line treatment with ipilimumab. The PBAC also considered that the submission underestimated the number of people exposed to dacarbazine or fotemustine by assuming unrealistic values around access and enrolment into clinical trials.
The PBAC further noted there were uncertainties with the dose of ipilimumab considering that the first-line trials use a higher dose of ipilimumab (10 mg/kg) whereas in the second-line setting the dose is 3 mg/kg and that higher doses might be used in clinical practice based on this first-line study.
The PBAC noted that no new clinical trials were presented. However, the submission provided additional analyses to support the effectiveness of ipilimumab in response to PBAC’s previous concerns. The PBAC noted that the analysis of the plateau effect in the survival curves (from Study CT-020) shows 13.7% survival at 2 years in the control arm of the ipilimumab studies. However, the plateau was less convincing when the two ipilimumab containing arms are pooled. The PBAC further noted that a plateau effect is reported for melanoma as a nature of the condition. The American Joint Committee on Cancer (AJCC) reports 10-20% of Stage IV patients are alive at 10 years, however, Balch et al (2009), shows that <10% of stage IV patients are alive at 10 years. Overall, the PBAC considered that there is some evidence of a plateau effect with ipilimumab treatment (at most providing up to 10% additional longer term survivors) but the durability of this effect remains unknown.
The PBAC noted that the submission presented an updated modelled economic evaluation including changes to the model structure, new utility weights, and extrapolation from the end of the Kaplan-Meier curves using a within-trial hazard approach (averaged using 3 years of data). The PBAC noted that the 10 year time horizon is retained despite the PBAC previously suggesting this is implausible. The PBAC agreed that the median overall survival (used in the previous submission) may underestimate the benefit of ipilimumab given the evidence that there is a plateau effect and accepted that the mean overall survival (7.08 months) is more appropriate. However, the PBAC remained unconvinced regarding the 10 year time horizon, given the low number of overall survivors at the conclusion of the trial, and that most patients had progressed by this stage. The PBAC considered a conservative estimate would be a time horizon of 5 years to account for the durable but not indefinite responders. The PBAC also noted that the choice of utility weights significantly favours ipilimumab.
Therefore, the PBAC considered that the base case ICER of between $45,000 and $75,000 per QALY gained is high and uncertain. The PBAC noted that the extrapolation method and point at which extrapolation occurs increases the ICER to between $75,000 and $105,000 per QALY gained and that sensitivity analyses show that the model is sensitive to a time horizon of 5 years and the choice of utility weights.
The PBAC considered that the utilisation and financial estimates for ipilimumab are highly uncertain due to potential use outside the requested restriction in the first-line clinical trial setting (dosed at 10 mg/kg compared to 3 mg/kg in the second line setting) and likely underestimation of uptake rates. The PBAC noted that the number of patients assumed to be treated with ipilimumab per year in the base case is lower than the number of patients diagnosed with unresectable Stage IIIC/IV melanoma each year, so the number of patients eligible for ipilimumab may potentially be higher.
The PBAC therefore rejected the submission because of uncertain extent of clinical benefit, uncertain clinical place in therapy and high and uncertain cost effectiveness.
In making this recommendation, the PBAC noted the consumer comments on this item.
Recommendation:
Reject
13. Context for Decision
The PBAC helps decide whether and, if so, how medicines should be subsidised in Australia. It considers submissions in this context. A PBAC decision not to recommend listing or not to recommend changing a listing does not represent a final PBAC view about the merits of the medicine. A company can resubmit to the PBAC or seek independent review of the PBAC decision.
14. Sponsor’s Comment
Bristol Myers Squibb is disappointed with the PBAC decision but remains committed to working with the PBAC to make Yervoy available on the PBS for eligible Australian patients with unresectable metastatic melanoma.




