Review of Statin Therapies
PDF printable version of this page (PDF 484 KB)
Purpose:
The Government accepted the following request made in the Senate on 22 November 2010
by Senator Xenophon (also representing Senator Fielding), to:
“Request advice from the Pharmaceutical Benefits Advisory Committee (PBAC) on any new evidence on whether or not two medicines, rosuvastatin and atorvastatin, should be included in the existing statins therapeutic group.”
Three reports were produced during the Review by two external consultancy groups engaged by the Department of Health and Ageing: preliminary report (December 2011), revised preliminary report (February 2012), and final report (23 May 2012).
Background:
The basis of the initial PBS listing of pravastatin, simvastatin and atorvastatin
was:
20mg simvastatin (40% reduction in LDL-C) = 20mg pravastatin = 10mg atorvastatin.
All three drugs were included in a Therapeutic Group.
In July 2005, the PBAC changed the basis of listing atorvastatin on the PBS from cost-minimisation to cost-effectiveness. The PBAC concluded and advised the Minister and the Pharmaceutical Benefits Pricing Authority that:
- Atorvastatin was more effective than simvastatin in lowering LDL-cholesterol (LDL-C).
- The relative price differential modelled in the submission’s cost effectiveness analysis was acceptable.
- Any further price change in simvastatin should not result in any increase in this price relativity.
- The only basis for judging whether the price relativity could be further increased would be an incremental cost effectiveness analysis based on major cardiovascular events measured directly in randomised trials rather than based on predictions modelled from surrogate outcomes.
As a consequence, atorvastatin was removed from the therapeutic group comprising simvastatin and pravastatin.
Rosuvastatin was recommended for listing at the July 2006 PBAC meeting on a cost-minimisation basis with atorvastatin, with the rosuvastatin to atorvastatin ratio of equi-effective doses being 1:3.
The statins-Higher Potency therapeutic group (atorvastatin, rosuvastatin) was formed in September 2009 as a separate group to the existing ‘statins’ therapeutic group (simvastatin, pravastatin).
These two therapeutic groups were in place until 1 April 2012, when following price reductions from price disclosure for simvastatin and pravastatin, the therapeutic group comprising these two F2 medicines was abolished. In addition, on 1 April 2012 the first new brand of atorvastatin was listed on the PBS. As a consequence, a 16% price reduction has been applied to atorvastatin, which has also moved to the F2 formulary. This 16% price reduction has also flowed onto rosuvastatin, which by way of being in the higher-potency therapeutic group with atorvastatin, has also moved to the F2 formulary. In summary, as of 1 April 2012, all four statins were in the F2 formulary, but only two, atorvastatin and rosuvastatin, are in a therapeutic group.
Below is a summary of the reports considered by the PBAC with the evidence included.
Methods of Clinical Review in the Reports
A systematic literature review was undertaken to collate any new evidence of the comparative safety and effectiveness of pravastatin, simvastatin, rosuvastatin and atorvastatin. A systematic search of the medical literature was conducted to identify relevant studies for all of the Terms of Reference (ToR). The search encompassed both primary and secondary prevention trials and was kept intentionally broader than the patient populations currently eligible for reimbursement on the PBS. This was primarily due to the complexity of patient eligibility on the PBS for statins and the consequential risk of missing relevant trials by using a restrictive search strategy. As a first step, the search strategy involved identifying a recent, good quality systematic review/meta-analysis that met the eligibility criteria for each of ToR 1, 2 and 3. These acted as a trial-finding tool. No specific search period was selected – searching began from October 2011 and proceeded retrospectively until the most recent, eligible systematic review/meta-analysis was identified. Once an eligible baseline systematic review was sourced, a series of literature searches (commencing from the search end-date in the systematic review) were conducted to identify recent published clinical trials. In this way all relevant trial data could be obtained and updated with “new” data (incorporating evidence published since the PBAC 2006 decision to list rosuvastatin). The comparative safety of statins in terms of both short-term and long-term adverse events (AEs) was assessed in ToR3 and different search strategies were employed to identify evidence on these AEs. Although only randomised controlled trials of short and long term duration were included to assess the comparative safety of the four statins, it was recognised that other safety considerations, such as pharmacologic differences, interactions and very rare AEs, would only be obtainable through observational evidence.
Eligible trials were critically appraised using standard methods to assess the risk of bias. Data were extracted for baseline population characteristics and standardised outcomes. Results were synthesised narratively and statistically and interpreted in terms of their clinical importance.
Comparative effectiveness was assessed primarily in terms of clinical outcomes, given PBAC’s stated preference to assess the comparative benefits of drugs in terms of treatment outcomes that are directly discernible and meaningful to patients. This approach was consistent with the requirements of ToR2. For completeness, and to address both ToR 1 and 2, information on the comparative impact of the four statins on the surrogate outcome of low density lipoprotein cholesterol (LDL-C) levels was provided. ToR3 was addressed by evaluating the comparative safety of the four statins, while ToR4 focussed on determining the net clinical benefit of each of the statins on the basis of the available comparative evidence. The clinical output of this ToR4 was not sufficient to develop an economic model to justify the price differentials across the identified dose comparisons. Therefore, ToR6 was addressed by providing additional context for PBAC decision-making – specifically by providing information on the long term safety and effectiveness of each of these statins relative to placebo; and by documenting the available evidence on cross-over and titration studies.
A key study identified in the literature search was the Cholesterol Treatment Trialists’ Collaboration (CTTC) individual patient data (IPD) meta-analysis published in 2005 (statin versus placebo / no treatment trials only), extended in 2010 (added head to head trials), and again updated in 2012 (compared statin effects across patients of varying baseline risk).
The CTTC 2010 publication was used as a baseline systematic review for addressing ToR2 – that is, the results in this Review were partly sourced from the CTTC publication.
Results of Clinical Review in the Reports
Term of Reference 1
|
Term of reference 1
|
The evidence consisted of thirty-six head to head randomised controlled trials, comparing atorvastatin, pravastatin, rosuvastatin and simvastatin, in terms of change in LDL-C. These trials were selected on the basis that the populations were consistent with the population eligible for PBS-reimbursed statins.
Results of the head-to-head comparison of LDL-C reduction from baseline were as follows:
- For comparisons of different doses of the same statin (‘within-statin’), higher dose treatment arms were associated with a greater LDL-C reduction than the lower dose treatment arms. Estimates of the difference ranged from a 14% greater reduction from baseline in patients receiving pravastatin 20mg compared with pravastatin 10 mg, to a 1.5% greater reduction from baseline when comparing atorvastatin 20 mg with atorvastatin 10 mg.
- When differences in LDL-C change from baseline between arms were pooled for the trials in each comparison, higher dose treatment had more effect than lower dose treatment, by between 3% and 7%, with no obvious exceptions.
- For the comparisons of different statins, simvastatin 5 mg, 10 mg and 20 mg had more effect than pravastatin 10 mg, 20 mg and 40 mg, respectively, in terms of LDL-C reduction from baseline by between 1% and 8%
- Rosuvastatin 5 mg, 10 mg 20 mg and 40 mg usually had more effect than atorvastatin 10 mg, 20 mg, 40 mg and 80 mg, respectively. However some studies comparing rosuvastatin 10 mg with atorvastatin 20 mg and rosuvastatin 40 mg with atorvastatin 80 mg reported a greater mean LDL-C reduction from baseline for atorvastatin.
Term of Reference 2
|
Term of reference 2 |
In 2005, the Cholesterol Treatment Trialists’ Collaboration (CTTC) published an individual patient data (IPD) meta-analysis of 14 randomised statin versus control trials examining cardiovascular endpoints. In 2010, an updated IPD meta-analysis, conducted by the CTTC, of 21 randomised statin versus control trials and 5 randomised ‘more’ versus ‘less intensive’ statin regimens was published. The primary aims of the CTTC meta-analyses were to report on the effects of lowering cholesterol on: total mortality, mortality associated with coronary artery disease and mortality from other causes. These aims had implications for the pre-specified objective of Term of Reference 2 (ToR2).
All 5 head-to-head randomised trials of ‘more’ versus ‘less intensive’ statin regimens (PROVE-IT, Phase Z of the A to Z Study, Treating to New Targets (TNT), IDEAL and SEARCH), included in the CTTC 2010 meta-analysis, were identified and included to address ToR2. However, only 11 of the statin versus control (placebo and/or usual care) trials, considered by the CTTC 2010 analyses, met the eligibility criteria specified for the reports. Most of these trials were ineligible because they reported on a different statin (e.g. fluvastatin, lovastatin) or the trial populations were not representative of the PBS eligibility criteria (i.e. primary prevention populations without adequate risk factors or a renal dialysis population). As a consequence, the pooled analyses presented by the CTTC, and examining the impact of lowering LDL-C on cardiovascular outcomes, could not be used.
To adequately explore a relationship between LDL-C reduction and cardiovascular risk in the manner requested by ToR2, an IPD multivariate regression analysis would be required. This analysis would need to specifically include trials relevant to the PBS population, and adequately control for prognostic covariates. Cardiovascular risk would need to be measured in a standardised manner across the trials. In the absence of these data, a trial-based approach was undertaken, supplemented by exploratory study-level meta-analyses that used CTTC defined and standardised cardiovascular outcomes.
Five head-to-head long-term randomised controlled trials demonstrated the impact of high versus low dose statins on clinical outcomes in populations consistent with those eligible for PBS-reimbursed statins. The statins and doses compared are as follows:
- PROVE-IT (N=4162): atorvastatin 80 mg versus pravastatin 40 mg;
- IDEAL (N=8888): atorvastatin 80 mg versus simvastatin 20 mg;
- TNT (N=10,001): atorvastatin 80 mg versus atorvastatin 10 mg;
- A to Z (N=4,497): placebo (4 months) then simvastatin 20 mg versus simvastatin 40 mg (1 month) then simvastatin 80 mg; and
- SEARCH (N=12,064): simvastatin 80 mg versus simvastatin 20 mg.
The higher doses examined in the head-to-head trials were predominantly higher than those used in the placebo-controlled trials (see Term of Reference 6).
Results of the long-term head-to-head randomised controlled trials were as follows:
- Atorvastatin 80 mg was more effective in terms of major vascular events (composite outcome) and coronary revascularisations than any of the lower dose statins (pravastatin 40 mg, simvastatin 20 mg and atorvastatin 10 mg);
- Using the CTTC defined endpoints (2010), the evidence in terms of deaths due to coronary heart disease, observed in the two simvastatin 80 mg versus 20 mg trials, remained unclear. For the A to Z trial, there was a statistically significant 43% risk reduction in deaths due to coronary heart disease favouring the simvastatin 80 mg arm (relative risk (RR) 0.57, 95% CI 0.35, 0.92), however this was not the case for the SEARCH trial (RR=0.99, 95% CI 0.86, 1.13). The SEARCH trial demonstrated a statistically significant 14% risk reduction in non-fatal MI favouring the simvastatin 80 mg arm (RR 0.86, 95% CI 0.75, 0.98), however this was not the case for the A to Z trial where there was no statistically significant difference, in risk of non-fatal MI, between the simvastatin 80 mg and 20 mg treatment arms (RR 0.96, 95% CI 0.76, 1.20);
- In some cases, the largest incremental mean LDL-C reduction from baseline resulted in the smallest observed point estimate of clinical benefit and vice versa. It was therefore plausible that there may be multiple causes of differences in treatment effect across trials, which may not be exclusively due to differences in LDL-C change. Given differences in trial populations and the inability to adjust for these other confounders or potentially explanatory variables, no conclusive generalisation can be made on a correlation between incremental LDL-C reduction (between high and low dose statins) and relative clinical benefit by comparing results across the different trials;
- No data on cardiovascular clinical endpoints were available comparing pravastatin and simvastatin; and
- No data with cardiovascular clinical endpoints were available comparing rosuvastatin with any of the other statins.
Term of Reference 3
|
Term of reference 3 |
Short-term safety
A total of 25 studies formed the evidence base to assess comparative statin safety.
All dose comparisons pre-specified for Term of Reference 1 were addressed, with the
exception of the following comparisons:
- simvastatin 40 mg vs pravastatin 80 mg;
- simvastatin 5 mg vs simvastatin 10 mg;
- simvastatin 40 mg vs simvastatin 80 mg;
- pravastatin 40 mg vs pravastatin 80 mg; and
- atorvastatin 20 mg vs simvastatin 80 mg.
Overall, event rates were very low. Withdrawal due to adverse events was reported by 17 included trials, representing 18 different comparisons. Due to the low numbers of patients withdrawn, no compelling conclusion can be reached regarding differences between the arms of the presented comparisons. Other points in relation to the adverse events identified are as follows:
- Data on deaths were too sparse to draw any conclusions (2 deaths amongst atorvastatin 10 mg patients, 1 death amongst simvastatin 20 mg patients);
- Increases in alanine transaminase to greater than three times normal were uncommon (<1%) for any statin or dose with the exception of atorvastatin 80 mg in which six patients (1.4% - 1.5%) reported an increased ALT;
- Increases in creatine kinase were commonly reported among the included trials, although it was rare for creatine kinase to be substantially elevated;
- No cases of myopathy were observed for any statin at any dose. Myopathy is more common in subjects with particular characteristics (such as concomitant medications, and coexisting illnesses) and these are likely to be under-represented in the trial populations; and
- Rhabdomyolysis was reported by only one trial and no events were reported for any arm. Duration of the included trials may have been insufficient to capture statin-induced rhabdomyolysis.
Long-term safety
Four meta-analyses, using either direct or indirect methods, were identified that
examined the comparative safety of statins
Preiss et al, 2011 conducted a meta-analysis of five randomised head to head trials which compared intensive-dose and intermediate-dose statins in patients without diabetes at baseline. The 5 head to head trials included a total of 32,752 patients and had a weighted mean follow-up of 4.9 years. They found the following:
- 2,749 subjects (8.4%) developed incident diabetes;
- In the combined data set, 149 more cases of incident diabetes occurred in patients assigned to intensive therapy than in those receiving moderate therapy (OR: 1.12; 95% CI: 1.04, 1.22); and
- There was no significant heterogeneity between studies for the occurrence of new-onset diabetes (I2=0%). However, the confidence interval for the I2 did not exclude substantial heterogeneity (0%, 79%).
Mills et al examined the safety and effectiveness of statins in a meta-analysis of 10 randomised controlled trials. Studies had to compare a statin of moderate dose with the same statin, or another statin, of higher dose and be of at least 6 months duration. The 10 trials enrolled a total of 41,778 participants. Results included:
- No evidence of increased cancer risk associated with intensive dose statin treatment over moderate dose statin treatment (k=6; 826 events vs 865 events, RR: 0.95; 95% CI: 0.87, 1.04, p=0.31; I2=0%).
- No increased incidence of rhabdomyolysis (k=6; 16 events vs 7 events, RR: 1.70; 95% CI: 0.56, 5.19, p=0.31; I2=20%). The results indicated a trend towards an increased risk but it was likely that the analysis lacked adequate power given the rare occurrence of rhabdomyolysis.
- Increased risk of AST beyond normal, associated with intensive dose statin treatment, with moderate heterogeneity across the trials (k=6; 67 events vs 19 events, RR: 3.15; 95% CI: 1.31, 7.54, p=0.01, I2=53%). The results were similar for increased ALT levels beyond normal, although there was substantial statistical heterogeneity when results were pooled (I2=93%). There was also a statistically significant increase in risk of CK beyond normal (203 events vs. 100 events, RR 2.86, 95% CI, 2.02, 4.04, p≤ 0.001), associated with intensive statin dosing.
Term of Reference 4
|
Term of reference 4 |
Table 1 provides a balance sheet of the number needed to treat to benefit from a high intensity statin rather than a low intensity statin, along with the number needed to harm as a consequence of each comparison. The net clinical benefit from a more intensive versus less intensive statin will depend on the population in which it is used and the duration of therapy. In the TNT trial, for example, patients who received atorvastatin 80 mg would be trading off a reasonable likelihood of preventing revascularisation and a moderate likelihood of preventing a non-fatal myocardial infarction against a moderate likelihood of developing a chronic disease (i.e. diabetes) in a period of 4.9 years. The baseline risk of a contemporary Australian population eligible for statin use may differ from that in the presented trials, although the trials were selected to be consistent with the population eligible for PBS-subsidised atorvastatin, pravastatin, simvastatin and rosuvastatin.
Table 1 - Number needed to treat to avoid one additional event or incur one additional event
|
Number Needed To Treat to Benefit (NNT)a [95% CI] |
Number Needed to Treat to Harm (NNH) [95% CI] |
|||||||||||
|
Trial |
Arms |
Follow up |
Final acc date |
LDL-C diffa |
SS |
% not diab |
Death from CHD |
Nonfatal MI |
Revascularis-ation |
Stroke |
Incident diabetesb |
Rhabdo-myolysisc |
|
PROVE IT |
A80 v |
2 |
2001 |
0.65 |
4162 |
82% |
220 |
113 |
41 |
NNH 1258 |
1928 |
∞ |
|
IDEAL |
A80 v |
4.8 |
2001 |
0.55 |
8888 |
84% |
1707 |
83 |
27 |
196 |
123 |
NNT 3698 |
|
A to Z |
S80 v |
1.97 |
2003 |
0.3 |
4497 |
78% |
115 |
371 |
331 |
265 |
103 |
589 |
|
SEARCH |
S80 v |
6.7 |
2001 |
0.39 |
12064 |
89% |
1238 |
92 |
152 |
252 |
142 |
771 |
|
TNT |
A80 v |
4.9 |
1999 |
0.62 |
10001 |
76% |
388 |
78 |
21 |
133 |
63 |
NNT 3795 |
NNT=number needed to treat with a higher intensity statin to avoid one additional
event than when treated with a lower intensity statin; NNH=number needed to treat
with a higher intensity statin to incur one additional event than when treated with
a lower intensity statin; Follow up=duration of trial in years; LDL-C diff=mmol/L
greater reduction of LDL-C at 1 year in the more intensive statin arm compared with
the less intensive statin arm; SS=sample size; death from CHD=death definitely or
probably as a consequence of coronary heart disease; MI=myocardial infarction; Final
acc date=final date of trial accrual; NS=not significant; Revascularisation refers
only to coronary revascularisation; % not diab is the percentage of patients in each
study that are not diabetic at baseline – this will affect the calculation of incident
diabetes.
a Data sourced from CTTC 2010 2. b Data sourced from Preiss et al 2011 12. Population
at risk of diabetes is given in ‘% not diab’ column
c Data sourced from individual studies. Point estimates are NNT or NNH depending
upon the column headings, unless otherwise specified and italicised.
Term of Reference 5
|
Term of reference 5 |
ToR5 described that, based on the clinical evidence on corresponding titration of each of the daily doses as specified in ToR1, the incremental costs incurred should be estimated. No head-to-head clinical endpoint evidence was available for the comparisons described in ToR1. Therefore, there was no direct basis to develop an economic model to justify the price differentials across the identified titrations.
As no direct clinical endpoint comparisons were available for ToR5, the economic model and associated translation issues were focussed on ToR6. In addition to trial-based evaluation, modelled evaluations based upon mixed treatment comparison (MTC) have been presented. The modelled economic evaluations report both a survival and a quality of life gain, and were therefore a cost-effectiveness and cost-utility analysis.
Term of Reference 6
|
Term of reference 6 |
Clinical
Thirteen long-term randomised placebo-controlled trials were considered for Term of
Reference 6 which provided additional efficacy evidence for statins. Available doses
in these trials were pravastatin 20 mg [k=1] and 40 mg [k=5], simvastatin 20 mg [k=1]
and 40 mg [k=1], atorvastatin 10 mg [k=2] and 80 mg [k=1], and rosuvastatin 10 mg
[k=2]. Trial populations were predominantly secondary prevention (i.e. previous cardiovascular
or cerebrovascular event) or high risk primary prevention, consistent with the populations
eligible for statins on the PBS.
Results from the long-term placebo-controlled trials were as follows:
- Overall, four of the five pravastatin 40 mg vs control trials demonstrated a statistically significant difference in major vascular events, major coronary events and coronary revascularisations favouring pravastatin 40 mg over control. When pooled, pravastatin also showed a significant reduction in strokes and all-cause mortality, albeit affected by substantial heterogeneity;
- Both simvastatin vs control trials demonstrated a statistically significant difference in major vascular events, major coronary events, coronary revascularisations and all-cause mortality favouring simvastatin over control. The HPS trial showed that simvastatin 40 mg was associated with a significant 24% reduction in fatal or non-fatal strokes;
- All three atorvastatin trials demonstrated a statistically significant risk reduction, favouring the atorvastatin arm, in terms of major vascular events, major coronary events, coronary revascularisation (except CARDS) and stroke. No atorvastatin trial demonstrated a statistically significant risk reduction favouring atorvastatin in terms of all-cause mortality;
- Despite substantial reductions, compared with placebo, of LDL-C at 1 year following randomisation, rosuvastatin 10 mg daily did not significantly alter the proportion of major vascular events, major coronary events, strokes or all-cause mortality in patients with heart failure over the 2.7 and 3.9 years median follow up for the CORONA and GISSI-HF trials, respectively.
Methods of Economic Analyses in the Reports
Mixed treatment comparison
A mixed treatment comparison (MTC) was undertaken for each of the outcomes (CHDD,
non-fatal MI, stroke and CR) using the trial data in the clinical evidence base. The
preferred source of evidence was the event rates from the supplementary web-appendix
to the CTTC (2010) publication. The impact of important prognostic factors on treatment
comparisons was investigated by regression modelling of the treatment effects from
each trial according to study level covariates. The regression was modelled on the
log odds ratio scale, which implied a linear relationship between the log odds ratio
and study-level covariates. The mixed treatment analysis assumed that more than one
treatment arm can come from an individual trial. Mixed treatment comparison regression
analyses were performed using the WinBUGS program which adopts Markov chain Monte
Carlo simulation techniques for model estimation. All analyses were performed with
three chains, where each was run for 20,000 iterations after a burn-in of 20,000 iterations
and demonstrated satisfactory convergence to their supporting posterior distributions.
Heterogeneity between trials was assessed using the standard deviation of the trial-specific
random treatment effects (s).
Economic analysis
Trial-based evaluations and a modelled evaluation based on the mixed treatment comparison
(“base-case”) were presented. The clinical outcomes included in the model were non-fatal
MI, CHDD, stroke and coronary revascularisation, as these outcomes were consistently
reported in the included clinical trials.
The trial-based evaluations reported the incremental cost per event averted for each trial. The modelled economic evaluations reported both a survival and a quality of life gain, and were therefore a cost-effectiveness and cost-utility analysis.
The economic evaluation was focused on particular statin doses as opposed to all possible statin doses or simply for each molecule. Whilst it was agreed that for completeness and for testing model specifications, including an analysis for all statin doses and testing at a molecule level would have been desirable, clinical outcome data were only available for a few strengths of each statin. Consequently, it was impossible to estimate or utilise efficacy for statin doses for which no CV outcome data exists. Further, the statin doses for which there were data represented only a proportion of doses for each statin. As such it was considered inappropriate to consolidate the available data for each statin product to represent all strengths of that statin.
Trial-based evaluation
The trial-based evaluations used the counts of CVD events reported from the included
trials. Costs for statins and total costs associated with CVD events were applied
to generate an incremental cost per event averted, for each type of CVD event.
Modelled evaluation
A deterministic decision-analytic Markov model was constructed in TreeAge Pro® 2009.
Excel® 2007 was used for data transformations prior to their input into the model.
The outcomes used in the modelled economic evaluation were quality-adjusted life-years
(QALYs), life-years gained (LYGs) and counts of each cardiovascular event. These
were final outcomes based on the health outcomes published in the literature and not
from transformations of surrogate outcomes, such as LDL-C levels. Transition probabilities
between health states were derived from the MTC synthesis. The modelled time horizon
was the rest of life (age 100 years) based on an initial age of 58 years.
The model included ten health states:
- at risk (on treatment) ,
- at risk (discontinued),
- post-stroke (on treatment),
- post-stroke (discontinued),
- first month post-MI,
- post-MI (on treatment),
- post-MI (discontinued),
- post-coronary revascularisation (on treatment),
- post-coronary revascularisation (discontinued), and
- dead.
Figure 1 illustrates the health states and possible transitions in the model.
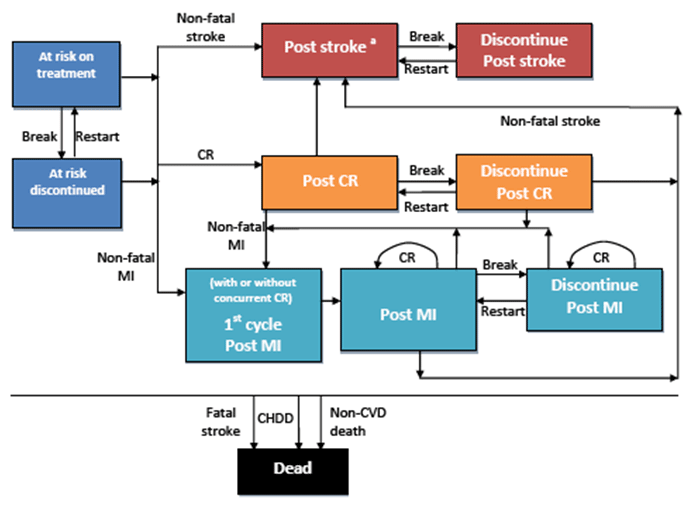
CR=coronary revascularisation; MI=myocardial infarction; break=discontinuation or
treatment break; CHDD = cardiovascular heart disease death
Not shown: Continue in current health state with no event
a From the Post stroke event patients can only move to discontinue post-stroke or
one of the dead health states. From the discontinue health state, patients can only
move to post-stroke and dead health states.
Data to inform economic evaluations
Trial based evaluation
Trial based evaluations presented in the reports estimated the cost per incident avoided
and were primarily determined by the cost of statin treatment and the estimate of
the total cost of clinical events avoided.
Statin costs
The cost of statin treatment, including an adjustment for a compliance rate of 84.2%
and including associated monitoring costs was used. Compliance was estimated using
Medicare data (10% sample) to estimate the number of scripts per patient, adjusting
for initiating and discontinuing treatment.
Treatment of CVD events costs
Table 2 presents the costs associated with CVD events in the trial based evaluations
and the modelled evaluation
Table 2 - CVD event costs used in the model
Event |
Cost per cycle |
|
Non-fatal MI – without PTCA (first 30 days) |
$6,641 a |
|
Non-fatal MI – with PTCA (first 30 days) |
$11,526 |
|
Non-fatal MI (period 2-12 inclusive) |
$346 |
|
Non-fatal MI (period 13+) |
$160 |
|
CHDD (one off) |
$3,423 |
|
Non-fatal stroke (acute) |
$11,984 |
|
Non-fatal stroke (period 1-12 inclusive) |
$1,407 |
|
Non-fatal stroke (period 13+) |
$366 |
|
Fatal stroke (one off) |
$2,440 |
|
Coronary revascularisation (acute) |
$20,052 |
|
Coronary revascularisation (ongoing) |
$160 |
|
Non-CVD death (one off) |
$3,423 |
PTCA=percutaneous transluminal coronary angioplasty; MI=myocardial infarction;
CHDD=coronary heart disease death; CVD=coronary vascular disease
a Includes cost for hospitalisation and 1/12 of the first year of monitoring cost.
Modelled economic evaluation
The modelled economic evaluation was a cost-effectiveness/cost-utility analysis and
presented outcomes for survival and quality of life. In order to conduct this analysis
a number of pre-modelling issues were identified and addressed according to Section
C of the 2008 PBAC Guidelines.
Pre-modelling studies
Table 3 presents a summary of the translation issues identified for the purposes of
the economic model addressing ToR 6.
Table 3 - Summary of results of pre-modelling studies and their uses in the economic evaluation
Pre-modelling study |
Results |
|
Applicability pre-modelling studies |
|
|
Patient demographics in PBS population |
Clinical trial data mean age 58-64 years, 75-83% male PBS data 2010/2011 by age and sex: |
|
Baseline risk of CVD events in at risk population (without statin treatment) |
Placebo arm of HPS trial is most applicable to the PBS population. Events rates adjusted for age and sex distribution in PBS population. The 30-day probabilities for each event by age category presented in Table 82 of the Review. |
|
Compliance to treatment |
Using Medicare data (10% sample) to estimate the number of scripts per patient, adjusting
for initiating and discontinuing treatment, the number of scripts per year is 10.26
per patient year. This may include a change in treatment (i.e. up-titration, down-titration
or switch). |
|
Extrapolation pre-modelling studies |
|
|
Extrapolation efficacy beyond trial duration |
From extension trials and cohort studies efficacy appears to be related to adherence,
with continued efficacy for good adherers and no difference in efficacy for poor adherers. |
|
Transformation pre-modelling studies |
|
|
Meta-analyses relevant comparisons |
MTC approach taken. Also includes |
|
Discontinuing and restarting |
Medicare data for patients initiating treatment in 2008/2009 and followed until 2011
was used for discontinuing and restarting treatment, with individual probabilities
for each individual statin/strength included in the economic model. |
|
Onset of efficacy with start of statin treatment and delay in offset after discontinuation treatment |
Although CTTC 2005 shows that risk the reduction of major vascular events was less
than half the reduction in subsequent years, no adjustment was made for delay in efficacy.
Efficacy was assumed to start immediately. |
|
Non-CVD mortality |
Age-specific mortality from ABS life tables was adjusted for CVD mortality from AIHW GRIM Books 2007. Values in Attachment C.2 of the Review were used. |
|
Utilities |
Age specific utilities used; disutility for non-fatal MI (acute and long-term), non-fatal
stroke and coronary revascularisation (acute) used. |
|
Other translation premodelling studies |
|
|
Costs of CVD events |
Acute, year 1 and year 2+ costs for CVD events included, costs also included for CHDD,
fatal stroke, and other cause mortality. |
|
Safety |
Very limited comparative safety data available. |
RR=relative risk; AE=adverse events; CVD=cardiovascular death; PBO=placebo; CHDD=coronary
heart disease death; AIHW=Australian Institute of Health and Welfare; ABS=Australian
Bureau of Statistics; CI=confidence interval; MI=myocardial infarction; HPS=Heart
Protection Study
a low potency (LP), includes all strengths of simvastatin (20 mg, 40 mg, and 80 mg)
and pravastatin (20 mg and 40 mg) included in the clinical trials
b high potency (HP) includes all strengths of atorvastatin (10 mg and 80 mg) and rosuvastatin
(10 mg) included in the clinical trials
c low-medium dose includes all doses, except for the highest available dose for each
statin (simvastatin 20 mg and 40 mg, pravastatin 20 mg and 40 mg, atorvastatin 10 mg,
rosuvastatin 10 mg)
d high dose includes all the highest strength of each statin (simvastatin 80 mg and
atorvastatin 80 mg)
Utilities
The utility/disutility values used in the modelled economic evaluations are presented
in Table 4 below.
Table. 4 - Utility/disutility values used in the model
|
Baseline utility |
Disutility first 30 days post-event |
Disutility days 30+ post-event |
|
|
<65 years |
0.8275 |
||
|
Non-fatal MI |
0.0051 |
0.0030 |
|
|
Non-fatal stroke |
0.0154 |
0.0154 |
|
|
Coronary revascularisation |
0.0016 |
0.0000 |
|
|
65-69 years |
0.7850 |
||
|
Non-fatal MI |
0.0051 |
0.0030 |
|
|
Non-fatal stroke |
0.0209 |
0.0209 |
|
|
Coronary revascularisation |
0.0016 |
0.0000 |
|
|
70-74 years |
0.7550 |
||
|
Non-fatal MI |
0.0051 |
0.0030 |
|
|
Non-fatal stroke |
0.0308 |
0.0308 |
|
|
Coronary revascularisation |
0.0016 |
0.0000 |
|
|
≥75 years |
0.6700 |
||
|
Non-fatal MI |
0.0051 |
0.0030 |
|
|
Non-fatal stroke |
0.0386 |
0.0386 |
|
|
Coronary revascularisation |
0.0016 |
0.0000 |
Source: Section C.3.5 of the Review
MI=myocardial infarction
Mixed Treatment Comparison (MTC) estimates for base case model
The numbers of non-fatal MIs, CHDDs, strokes and coronary revascularisation were extracted
from the CTCC publication, by preference. For CORONA and SPARCL, the data was extracted
from the relevant publications. The numbers of events used for the MTC are presented
in Table 5 and Table. 6.
Table 5 - Number of non-fatal MIs and CHDDs in the included clinical trials used for the mixed treatment comparison– from CTTC (2010) a
Trial |
Comparison |
Non Fatal MI |
CHDD |
||
|
statin |
control |
statin |
control |
||
|
n/N |
n/N |
n/N |
n/N |
||
|
PBO controlled trials |
|||||
|
GISSI-P |
P20 vs NT |
38/2138 |
40/2133 |
7/2138 |
19/2133 |
|
PROSPER |
P40 vs PBO |
222/2891 |
254/2913 |
94/2891 |
122/2913 |
|
ALLHAT-LLT |
P40 vs UC |
229/5170 |
276/5185 |
160/5170 |
162/5185 |
|
LIPID |
P40 vs PBO |
310/4512 |
408/4502 |
69/4512 |
116/4502 |
|
CARE |
P40 vs PBO |
135/2081 |
173/2078 |
40/2081 |
56/2078 |
|
WOSCOPS |
P40 vs PBO |
143/3302 |
204/3293 |
18/3302 |
35/3293 |
|
SSSS |
S20 vs PBO |
285/2221 |
436/2223 |
111/2221 |
189/2223 |
|
HPS |
S40 vs PBO |
357/10269 |
574/10267 |
375/10269 |
475/10267 |
|
ASCOT-LLA |
A10 vs PBO |
82/5168 |
130/5137 |
41/5168 |
46/5137 |
|
CARDS |
A10 vs PBO |
27/1428 |
44/1410 |
21/1428 |
26/1410 |
|
SPARCL |
A80 vs PBO |
43/2365 |
82/2366 |
40/2365 |
39/2366 |
|
CORONA |
R10 vs PBO |
116/2514 |
145/2497 |
15/2514 b |
9/2497 b |
|
GISSI-HF |
R10 vs PBO |
51/2285 |
58/2289 |
14/2285 |
21/2289 |
|
Statin vs Statin trials |
high dose |
low dose |
high dose |
low dose |
|
|
A to Z |
S80 vs S20 |
138/2265 |
142/2232 |
26/2265 |
45/2232 |
|
SEARCH |
S80 vs S20 |
397/6031 |
463/6033 |
379/6031 |
384/6033 |
|
PROVE IT |
A80 vs P40 |
130/2099 |
146/2063 |
22/2099 |
31/2063 |
|
IDEAL |
A80 vs S20 |
267/4439 |
321/4449 |
175/4439 |
178/4449 |
|
TNT |
A80 vs A10 |
243/4995 |
308/5006 |
43/4995 |
56/5006 |
MI = myocardial infarction; CHDD = cardiovascular heart disease death; NT = normal
treatment; UC = usual care; PBO = placebo; A = atorvastatin; S = simvastatin; P =
pravastatin; R = rosuvastatin
a All input parameters derived from CTTC, except for CORONA and SPARCL which were
derived from the individual trial publications.
b In the previous analyses, for CORONA the values for CHDD were for cardiovascular
deaths. This is likely an overestimate. The values have been updated and include now
only fatal MIs. While this is not the ideal value, as it may exclude many CHDD, it
is likely to better reflect CHDD.
Table. 6 - Number of strokes and coronary revascularisations in the included clinical trials used for the mixed treatment comparison – from CTTC (2010) a
Trial |
Comparison |
Stroke |
CR |
||
|
statin |
control |
statin |
control |
||
|
n/N |
n/N |
n/N |
n/N |
||
|
Placebo controlled |
|||||
|
GISSI-P |
P20 vs NT |
20/2138 |
19/2133 |
155/2138 |
174/2133 |
|
PROSPER |
P40 vs PBO |
135/2891 |
131/2913 |
39/2891 |
48/2913 |
|
ALLHAT-LLT |
P40 vs UC |
209/5170 |
231/5185 |
303/5170 |
344/5185 |
|
LIPID |
P40 vs PBO |
169/4512 |
204/4502 |
584/4512 |
706/4502 |
|
CARE |
P40 vs PBO |
52/2081 |
76/2078 |
294/2081 |
391/2078 |
|
WOSCOPS |
P40 vs PBO |
46/3302 |
51/3293 |
51/3302 |
79/3293 |
|
SSSS |
S20 vs PBO |
56/2221 |
76/2223 |
252/2221 |
383/2223 |
|
HPS |
S40 vs PBO |
452/10269 |
591/10267 |
513/10269 |
725/10267 |
|
ASCOT-LLA |
A10 vs PBO |
89/5168 |
121/5137 |
51/5168 |
81/5137 |
|
CARDS |
A10 vs PBO |
21/1428 |
39/1410 |
24/1428 |
34/1410 |
|
SPARCL |
A80 vs PBO |
265/2365 |
311/2366 |
NC b |
NC b |
|
CORONA |
R10 vs PBO |
103/2514 |
115/2497 |
119/2514 |
104/2497 |
|
GISSI-HF |
R10 vs PBO |
82/2285 |
66/2289 |
36/2285 |
48/2289 |
|
Statin vs Statin |
High dose |
Low dose |
High dose |
Low dose |
|
|
PROVE IT |
A80 vs P40 |
21/2099 |
19/2063 |
315/2099 |
360/2063 |
|
IDEAL |
A80 vs S20 |
151/4439 |
174/4449 |
579/4439 |
743/4449 |
|
A to Z |
S80 vs S20 |
28/2265 |
36/2232 |
119/2265 |
124/2232 |
|
SEARCH |
S80 vs S20 |
255/6031 |
279/6033 |
570/6031 |
610/6033 |
|
TNT |
A80 vs A10 |
117/4995 |
155/5006 |
667/4995 |
904/5006 |
MI = myocardial infarction; CHDD = cardiovascular heart disease death; NT = normal
treatment; UC = usual care; PBO = placebo; A = atorvastatin; S = simvastatin; P =
pravastatin; R = rosuvastatin; NC = not calculable; CR = coronary revascularisation
a All input parameters derived from CTTC, except for CORONA and SPARCL which were
derived from the individual trial publications.
b The values reported in Section B.6 for revascularisations for the SPARCL trial include
peripheral revascularisations. As this is not comparable to coronary revascularisations,
these values have been excluded from the mixed treatment comparison.
The odds ratios estimated using the MTC are provided in Table 7. The results from this MTC were used in the base case economic model.
Table 7 - Odds ratios produced by the MTC used in the base case economic model
Statin |
Median (95% CrI) |
||||
|
Non-fatal MI |
CHDD |
Stroke |
CR |
||
|
PBO Controlled |
|||||
|
Prava 20 |
PBO |
0.93 (0.58, 1.51) |
0.35 (0.12, 0.96) |
1.05 (0.51, 2.16) |
0.88 (0.62, 1.24) |
|
Prava 40 |
PBO |
0.76 (0.68, 0.84) |
0.75 (0.57, 0.98) |
0.86 (0.71, 1.02) |
0.75 (0.64, 0.86) |
|
Simva 20 |
PBO |
0.63 (0.53, 0.75) |
0.62 (0.39, 1.00) |
0.80 (0.56, 1.09) |
0.67 (0.51, 0.85) |
|
Simva 40 |
PBO |
0.61 (0.50, 0.74) |
0.78 (0.44, 1.40) |
0.75 (0.53, 1.07) |
0.69 (0.51, 0.93) |
|
Simva 80 |
PBO |
0.55 (0.44, 0.71) |
0.52 (0.26, 0.95) |
0.70 (0.43, 1.05) |
0.62 (0.44, 0.87) |
|
Atorva 10 |
PBO |
0.66 (0.54, 0.80) |
0.86 (0.55, 1.33) |
0.79 (0.58, 1.02) |
0.73 (0.55, 0.92) |
|
Atorva 80 |
PBO |
0.54 (0.46, 0.64) |
0.68 (0.46, 1.01) |
0.73 (0.56, 0.92) |
0.53 (0.42, 0.67) |
|
Rosuva 10 |
PBO |
0.81 (0.64, 1.02) |
0.99 (0.52, 1.92) |
1.03 (0.77, 1.41) |
1.00 (0.73, 1.33) |
|
LM dose a |
PBO |
0.71 (0.66, 0.78) |
0.75 (0.64, 0.88) |
0.85 (0.77, 0.94) |
0.76 (0.68, 0.85) |
|
High dose b |
PBO |
0.59 (0.52, 0.69) |
0.68 (0.52, 0.87) |
0.75 (0.64, 0.88) |
0.62 (0.52, 0.75) |
|
LP c |
PBO |
0.73 (0.66, 0.83) |
0.73 (0.60, 0.87) |
0.84 (0.75, 0.96) |
0.78 (0.68, 0.90) |
|
HP d |
PBO |
0.65 (0.56, 0.76) |
0.80 (0.62, 1.03) |
0.82 (0.71, 0.96) |
0.70 (0.58, 0.87) |
|
Statin vs statin comparisons |
|||||
|
Prava 40 |
Prava 20 |
0.82 (0.49, 1.31) |
2.14 (0.75, 6.54) |
0.82 (0.39, 1.70) |
0.85 (0.58, 1.24) |
|
Simva 20 |
Prava 20 |
0.68 (0.40, 1.13) |
1.79 (0.59, 5.85) |
0.75 (0.34, 1.66) |
0.76 (0.49, 1.17) |
|
Simva 40 |
Prava 20 |
0.66 (0.38, 1.10) |
2.23 (0.70, 7.58) |
0.72 (0.33, 1.58) |
0.79 (0.50, 1.25) |
|
Simva 80 |
Prava 20 |
0.60 (0.35, 1.02) |
1.48 (0.43, 5.08) |
0.66 (0.28, 1.52) |
0.71 (0.43, 1.14) |
|
Atorva 10 |
Prava 20 |
0.72 (0.42, 1.20) |
2.45 (0.81, 7.82) |
0.75 (0.35, 1.60) |
0.83 (0.52, 1.25) |
|
Atorva 80 |
Prava 20 |
0.59 (0.35, 0.97) |
1.93 (0.66, 6.15) |
0.69 (0.32, 1.47) |
0.61 (0.40, 0.92) |
|
Rosuva 10 |
Prava 20 |
0.88 (0.50, 1.47) |
2.81 (0.84, 10.1) |
0.98 (0.45, 2.15) |
1.13 (0.70, 1.77) |
|
Simva 20 |
Prava 40 |
0.83 (0.69, 1.02) |
0.83 (0.49, 1.42) |
0.93 (0.64, 1.33) |
0.89 (0.68, 1.16) |
|
Simva 40 |
Prava 40 |
0.80 (0.64, 1.00) |
1.04 (0.55, 2.00) |
0.88 (0.60, 1.32) |
0.92 (0.67, 1.30) |
|
Simva 80 |
Prava 40 |
0.73 (0.57, 0.95) |
0.69 (0.33, 1.32) |
0.81 (0.49, 1.28) |
0.83 (0.58, 1.18) |
|
Atorva 10 |
Prava 40 |
0.88 (0.70, 1.08) |
1.14 (0.69, 1.90) |
0.92 (0.65, 1.25) |
0.97 (0.73, 1.26) |
|
Atorva 80 |
Prava 40 |
0.72 (0.60, 0.86) |
0.90 (0.59, 1.41) |
0.85 (0.63, 1.13) |
0.71 (0.57, 0.90) |
|
Rosuva 10 |
Prava 40 |
1.07 (0.83, 1.39) |
1.31 (0.65, 2.71) |
1.20 (0.86, 1.74) |
1.33 (0.94, 1.84) |
|
Simva 40 |
Simva 20 |
0.97 (0.74, 1.26) |
1.25 (0.59, 2.64) |
0.95 (0.60, 1.56) |
1.03 (0.71, 1.56) |
|
Simva 80 |
Simva 20 |
0.88 (0.75, 1.04) |
0.83 (0.50, 1.25) |
0.88 (0.64, 1.16) |
0.93 (0.74, 1.17) |
|
Atorva 10 |
Simva 20 |
1.06 (0.83, 1.32) |
1.38 (0.75, 2.50) |
0.99 (0.66, 1.46) |
1.09 (0.79, 1.46) |
|
Atorva 80 |
Simva 20 |
0.86 (0.72, 1.03) |
1.09 (0.68, 1.73) |
0.92 (0.67, 1.26) |
0.80 (0.63, 1.03) |
|
Rosuva 10 |
Simva 20 |
1.29 (0.96, 1.72) |
1.58 (0.72, 3.53) |
1.30 (0.84, 2.09) |
1.49 (1.00, 2.18) |
|
Simva 80 |
Simva 40 |
0.91 (0.67, 1.25) |
0.67 (0.26, 1.51) |
0.92 (0.51, 1.57) |
0.90 (0.56, 1.39) |
|
Atorva 10 |
Simva 40 |
1.09 (0.82, 1.04) |
1.10 (0.53, 2.27) |
1.36 (0.87, 2.19) |
1.05 (0.69, 1.52) |
|
Atorva 80 |
Simva 40 |
0.89 (0.69, 1.04) |
0.87 (0.43, 1.76) |
0.96 (0.62, 1.45) |
0.77 (0.52, 1.12) |
|
Rosuva 10 |
Simva 40 |
1.33 (0.98, 1.04) |
1.26 (0.53, 3.02) |
1.36 (0.87, 2.19) |
1.44 (0.92, 2.15) |
|
Atorva 10 |
Simva 80 |
1.20 (0.89, 1.59) |
1.66 (0.82, 3.66) |
1.13 (0.69, 1.85) |
1.17 (0.78, 1.69) |
|
Atorva 80 |
Simva 80 |
0.67 (0.50, 0.89) |
0.69 (0.32, 1.47) |
0.71 (0.47, 1.03) |
0.54 (0.37, 0.80) |
|
Rosuva 10 |
Simva 80 |
0.98 (0.77, 1.24) |
1.30 (0.72, 2.65) |
1.04 (0.69, 1.64) |
0.86 (0.62, 1.20) |
|
Atorva 80 |
Atorva 10 |
0.82 (0.68, 0.99) |
0.79 (0.49, 1.29) |
0.92 (0.70, 1.26) |
0.73 (0.59, 0.95) |
|
Rosuva 10 |
Atorva 10 |
1.22 (0.90, 1.66) |
1.15 (0.53, 2.53) |
1.31 (0.89, 2.05) |
1.37 (0.92, 2.05) |
|
Rosuva 10 |
Atorva 80 |
1.50 (1.12, 1.99) |
1.45 (0.68, 3.16) |
1.42 (0.98, 2.14) |
1.86 (1.26, 2.69) |
|
High dose a |
LM dose b |
0.83 (0.74, 0.94) |
0.91 (0.71, 1.11) |
0.89 (0.77, 1.02) |
0.81 (0.70, 0.95) |
|
HP c |
LP d |
0.89 (0.75, 1.04) |
1.09 (0.85, 1.46) |
0.98 (0.82, 1.16) |
0.90 (0.74, 1.12) |
CrI=credible interval; CR=coronary revascularisation; MTC=mixed treatment comparison;
CHDD=coronary heart disease death; LP=low potency; LM = low-medium; HP=high potency;
Bold=statistically significant.
Values have changed due to changes in some of the input parameters for the Final Report.
a high dose includes all the highest strength of each statin (simvastatin 80 mg and
atorvastatin 80 mg)
b low-med dose includes all doses, except for the highest available dose for each
statin (simvastatin 20 mg and 40 mg, pravastatin 20 mg and 40 mg, atorvastatin 10 mg,
rosuvastatin 10 mg)
c high potency (HP) includes all strengths of atorvastatin (10 mg and 80 mg) and rosuvastatin
(10 mg) included in the clinical trials
d low potency (LP), includes all strengths of simvastatin (20 mg, 40 mg, and 80 mg)
and pravastatin (20 mg and 40 mg) included in the clinical trials
The PBAC noted the inconsistent results produced by the MTC approach across the various statins and their doses and considered that there was substantial heterogeneity in the included trials, which caused the indirect meta-analysis to be subject to random variation. As a consequence, differences observed were likely to be due to differences in patient populations and trial characteristics or random variation. An additional problem that also applied to the trial-based evaluation is the small numbers of cardiovascular events, resulting in statistically imprecise estimates of treatment effect.
Results of Economic Analyses in the Reports
Trial-based evaluations
A summary of the results from the trial-based evaluations is presented in Figure 2.
Figure 2 - Trial-based ICERs, comparator placebo
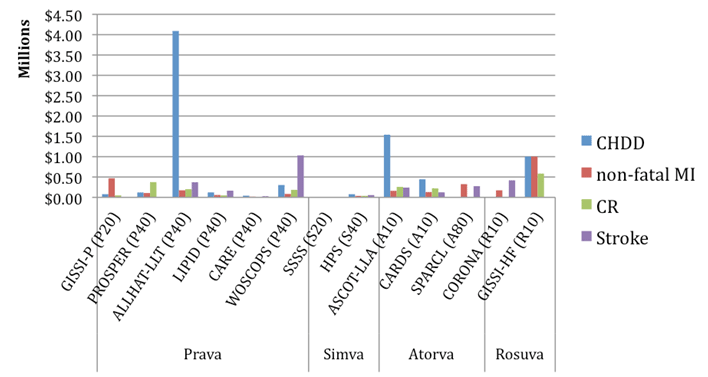
Atorva = atorvastatin; Prava = pravastatin; Rosuva = rosuvastatin; Simva = simvastatin;
CHDD=coronary heart disease death; MI=myocardial infarction; CR=coronary revascularisation;
Note: dominated and dominant results are not represented in this graph. As such this
may give some misleading impressions.
There was heterogeneity between trials due to differences in: population characteristics including age, gender, prior CHD, diabetes, current smokers and baseline LDL-C; trial design; and, trial duration as well as the year and place in which the trial was conducted. As such, comparisons between the results of the trial based evaluations were inconsistent and potentially misleading. For example, compared to placebo, pravastatin 40 mg had the lowest cost per stroke averted ($27,707 - CARE) but was dominated by placebo in another trial (PROSPER).
As the costs included in the trial-based economic evaluations are statin costs, monitoring costs and the costs for all included events (i.e. CHDD, non-fatal MI, coronary revascularisation and stroke), some differences between clinical outcomes of the trials become more apparent. For example, the relative numbers of non-fatal MIs prevented do not appear to differ significantly between the A to Z trial and the SEARCH trial (138 vs 142 for A to Z simvastatin 80 mg vs simvastatin 20 mg and 397 vs 463 for SEARCH simvastatin 80 mg vs simvastatin 20 mg; see Attachment D.5 of the Review). As the total cost has been used, i.e. statin and monitoring cost and cost saving due to avoided events, there is a difference between the A to Z and SEARCH trials, with a net cost for A to Z (statin + monitoring cost of $679,493 and a cost for events avoided of -$430,090; total cost: $249,403) and a cost saving for SEARCH (statin + monitoring cost of $2,435,521, cost for events avoided: -$2,927,674; total cost -$492,153). The resulting cost per non-fatal MI avoided for A to Z is $249,403/4 = $62, 351 and for SEARCH is -$492,153/66 = dominant.
Modelled economic evaluation – “base case”
Figures 3 and 4 plot the ICERs (QALYs) on the cost-effectiveness plane using the RRs from the MTC and with QALYs as the outcome measure.
These results are largely due to heterogeneity caused by the different characteristics of the patient populations in the trials as well as temporal and geographic differences between health services when and where the trials were conducted as well as random variation. As such, comparison of ICERs between the statins needs to be done with caution.
Within Figure 3, the solid line represents a hypothetical ICER threshold of $50,000 per QALY. Those statins to the right of this solid line have an ICER of less than $50,000 compared to placebo.
Figure 3-Incremental costs and QALYs for statins vs placebo – “base case”
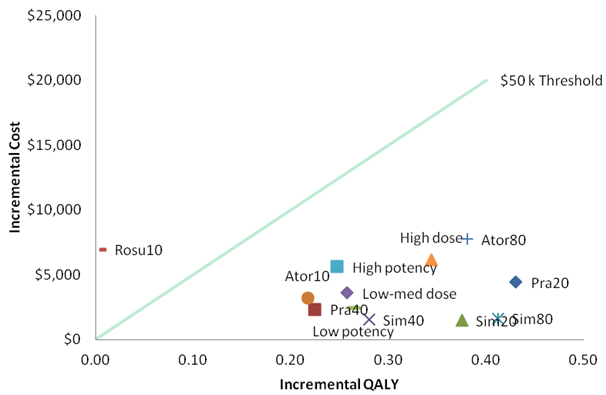
A10 = atorvastatin 10 mg; A80 = atorvastatin 80 mg; High dose = includes atorva 80 and simva 80; HP = high potency (includes atorva 10, atorva 80 and rosuva 10); LP = low potency (includes simva 20, simva 40, simva 80, prava 20, prava 40); Low-med dose = includes simva 20, prava 40 and atorva 10; P20 = pravastatin 20 mg; P40 = pravastatin 40 mg; S20 = simvastatin 20 mg; simvastatin 40 mg; R10 = rosuvastatin 10 mg
Figure 4 - Incremental costs and QALYs for statin vs statin comparisons – “base case”
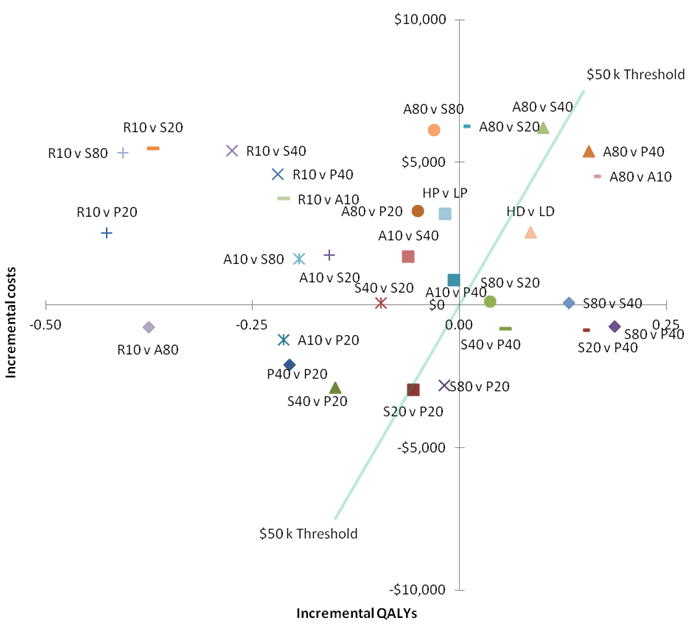
A10 = atorvastatin 10 mg; A80 = atorvastatin 80 mg; HD = high dose (includes atorva
80 and simva 80); HP = high potency (includes atorva 10, atorva 80 and rosuva 10);
LP = low potency (includes simva 20, simva 40, simva 80, prava 20, prava 40); LD =
low-medium dose (includes simva 20, prava 40 and atorva 10); P20 = pravastatin 20 mg;
P40 = pravastatin 40 mg; S20 = simvastatin 20 mg; simvastatin 40 mg; QALY = quality
adjusted life years; R10 = rosuvastatin 10 mg
Within Figure 4, the blue line represents a hypothetical ICER threshold of $50,000 per QALY. Within the north-east quadrant of Figure 4, those points to the right of this blue line represent a head to head comparison that results in an ICER of less than $50,000 per QALY. Within the north-west quadrant of Figure 4, all of those points represent a head to head comparison that results in a dominated ICER, that is, a scenario where the comparator is both more effective and less costly. Within the south-east quadrant of Figure 4, all of those points represent a head to head comparison that results in a dominant ICER, that is, a scenario where the proposed statin is both more effective and less costly than the comparator.
Based on the MTC, some counterintuitive incremental QALY results occur. For example, compared with placebo, simvastatin 40 mg resulted in fewer QALYs compared with simvastatin 20 mg. The differences in QALYs by drug strength were less pronounced than the differences between drugs. Pravastatin 40 mg produced the greatest QALYs, and rosuvastatin 10 mg the least with less than 0.01 QALYs gained compared with placebo. While there was an increase in QALYs gained for high dose, compared to low-medium dose there was no gain in QALYs for high potency statins compared to low potency statins. These results should be viewed in the context of the heterogeneity between the clinical trials including different characteristics of the patient populations in the trials as well as temporal and geographic differences between health services when and where the trials were conducted.
A number of sensitivity analyses were also conducted.
Table 8 provides the results of the multivariate sensitivity analyses around the atorvastatin 80 mg vs atorvastatin 10 mg comparison.
Table 8 Results of multivariate sensitivity analyses – atorvastatin 80 mg vs atorvastatin 10 mg
Analyses |
Δ Cost |
Δ QALYs |
ICER |
|
Atorva 80 vs atorva 10 |
$4,510 |
0.16 |
$27,656 |
|
Decrease price of low dose by 25% and 10 year efficacy cut-off |
$5,569 |
0.11 |
$51,795 |
|
Decrease price of high dose by 25% and 10 year efficacy cut-off |
$2,446 |
0.11 |
$22,747 |
|
Males, age 45 |
$4,910 |
0.23 |
$21,804 |
|
Males, age 75 |
$2,746 |
0.13 |
$20,891 |
|
Females, age 45 |
$5,402 |
0.21 |
$25,733 |
|
Females, age 75 |
$3,789 |
0.05 |
$84,038 |
|
HPS (75% males, aged 64) |
$4,149 |
0.19 |
$22,425 |
QALY=quality adjusted life years; HPS=Heart Protection Study; ICER=incremental cost effectiveness ratio; atorva = atorvastatin
As the model was sensitive to baseline risk and age of the patient, multivariate analyses were performed assuming 50% male for various combinations of age and baseline risk. The results are presented in Table 9.
Table 9 ICERs by age and baseline risk (atorvastatin 80 mg vs atorvastatin 10 mg)
5-year baseline risk of CVD event |
Age (years) |
||
|
45 |
58 |
75 |
|
|
Atorva 80 vs atorva 10a |
$16,413 |
$16,028 |
$13,803 |
|
10% |
$32,278 |
$34,237 |
$38,448 |
|
15% |
$19,648 |
$21,192 |
$24,328 |
|
20% |
$13,777 |
$14,994 |
$17,451 |
|
25% |
$10,531 |
$11,496 |
$13,467 |
|
30% |
$8,548 |
$9,319 |
$10,929 |
CVD=cardiovascular disease; ICER=incremental cost effectiveness ratio; QALY=quality
adjusted life year
a Age-specific 5-year risks (from 16.2% in ages <65 to 23.6% in ages ≥75)
red cells: ICER >$50,000/QALY; yellow cells: ICER >$30,000/QALY and ≤$50,000/QALY;
green cells: ICER is ≤30,000/QALY
For a cohort with a starting age of 58, the base case risk of CVD events produces an ICER of $16,028, which is similar to the ICER when the 5-year risk of CVD events is 20%. In the base case analysis, the risk of CVD events increases with age. For patients with a baseline 5-year risk of CVD events of less than 10%, the ICERs may be above $50,000/QALY.
Estimated PBS Usage and Financial Implications:
The figure below presents the percentages of patients on the different statins and strengths for patients initiating treatment and all patients in 2010/2011 financial year. Note that the results for the initiating patients are for a 10% cohort only.
Figure 5 - Percentage initiating and all patients on statins in 2010/2011 financial
year
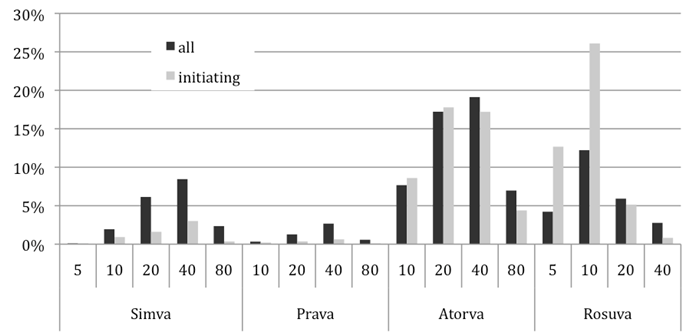
simva=simvastatin; prava=pravastatin; atorva=atorvastatin; rosuva=rosuvastatin
In the last financial year (2010/2011), more than 25% of the patients initiating statin treatment (defined as not having received any statin therapy in the last 24 months) started with rosuvastatin 10mg. Other common starting therapies were atorvastatin 20 and 40mg followed by rosuvastatin 5mg. Pravastatin and simvastatin were less common starting therapies in 2010/2011 (1.3% and 6.0% of patients starting, respectively). Currently, atorvastatin 40mg is the most commonly used therapy (19.1% of all scripts), followed by atorvastatin 20mg (17.2%) and rosuvastatin 10mg (12.2%). No clinical evidence is available for the simvastatin 5mg and 10mg, pravastatin 10mg and 80mg, atorvastatin 20 and 40mg and rosuvastatin 5mg, 20mg and 40mg in the current PBS population: however with publication of the SATURN Trial more information is now available, but not evaluated.
Compared to the participants in clinical trials statins are used to a greater extent in females (51% female in Australia)
Despite the lack of clinical comparison, outcomes utilisation data shows rapid uptake of higher strength and ‘potency’ statins, especially rosuvastatin.
Current use and financial cost – 2007-2011
Number of patients treated: an estimated total of up to 2.6 million in 2010/2011 for all statin prescriptions. Where combination medicines include a statin of interest to the review the number of prescriptions was included in analyses.
Number of prescriptions/packs dispensed: The number of scripts per statin and strength dispensed from 2006/07 to 2010/11 are presented in Table 10.
Table 10 - Number of PBS prescriptions dispensed from 2006/07 to 2010/11
Drug |
Strength |
2006/07 |
2007/08 |
2008/09 |
2009/10 |
2010/11 |
|
Prava |
10 |
130,748 |
113,848 |
99,967 |
87,572 |
79,605 |
|
20 |
550,351 |
471,010 |
400,594 |
346,821 |
304,555 |
|
|
40 |
1,088,656 |
953,858 |
834,886 |
723,020 |
637,744 |
|
|
80 |
123,143 |
140,824 |
147,093 |
143,909 |
138,478 |
|
|
Total |
1,892,898 |
1,679,540 |
1,482,540 |
1,301,322 |
1,160,382 |
|
|
Simva |
5 |
38,156 |
32,522 |
27,941 |
25,234 |
24,617 |
|
10 |
724,607 |
654,657 |
578,258 |
511,946 |
463,430 |
|
|
20 |
2,180,412 |
2,004,512 |
1,814,766 |
1,623,612 |
1,463,660 |
|
|
40 |
2,611,117 |
2,522,801 |
2,369,229 |
2,188,051 |
2,017,164 |
|
|
80 |
687,670 |
662,676 |
630,406 |
595,982 |
561,195 |
|
|
Total |
6,241,962 |
5,877,168 |
5,420,600 |
4,944,825 |
4,530,066 |
|
|
Atorva a |
10 |
1,989,816 |
1,966,325 |
1,926,532 |
1,870,046 |
1,830,329 |
|
20 |
3,647,428 |
3,906,471 |
4,070,931 |
4,104,905 |
4,105,701 |
|
|
40 |
3,273,274 |
3,775,971 |
4,176,243 |
4,425,051 |
4,559,593 |
|
|
80 |
1,168,685 |
1,319,857 |
1,470,944 |
1,582,826 |
1,663,840 |
|
|
Total |
10,079,203 |
10,968,624 |
11,644,650 |
11,982,828 |
12,159,463 |
|
|
Rosuva |
5 |
41,155 |
231,755 |
483,551 |
753,925 |
1,006,938 |
|
10 |
227,176 |
960,537 |
1,687,894 |
2,374,497 |
2,914,001 |
|
|
20 |
60,109 |
343,980 |
700,721 |
1,089,628 |
1,411,814 |
|
|
40 |
22,086 |
140,593 |
303,985 |
491,353 |
662,038 |
|
|
Total |
350,526 |
1,676,865 |
3,176,151 |
4,709,403 |
5,994,791 |
|
|
All statins |
18,564,589 |
20,202,197 |
21,723,941 |
22,938,378 |
23,844,702 |
|
|
% growth compared to 2006/07 |
100% |
109% |
117% |
124% |
128% |
|
Source: Medicare data and estimates of undercopayment prescriptions – DUSC database
a Includes scripts for atorvastatin with amlodipine
atorva=atorvastatin; simva=simvastatin; prava=pravastatin; rosuva=rosuvastatin
Figure 6 - Use of statins from 2006/07 until 2010/11
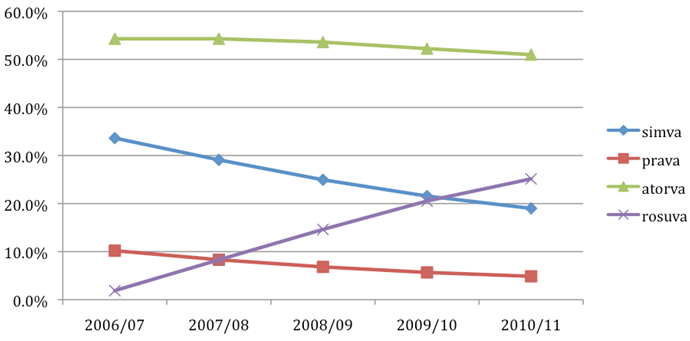
atorva = atorvastatin; prava = pravastatin; rosuva = rosuvastatin; simva = simvastatin
Since PBS listing in 2007, rosuvastatin has increased its market share to 25.1%, while the market share for other statins, in particular simvastatin and pravastatin, has decreased (from 33.6% to 19.0% and 10.2% to 4.9%, respectively). The total number of statin scripts in 2010/2011 financial year was 23.8 million.
The lower strengths of pravastatin and simvastatin are priced below the general copayment.
Table 11 - PBS/RPBS cost (in million $) for the last 5 financial years by statin
Drug |
2006/07 |
2007/08 |
2008/09 |
2009/10 |
2010/11 |
Total |
|
Simvastatin |
$309.44 |
$237.51 |
$170.99 |
$154.64 |
$140.03 |
$1,012.61 |
|
Pravastatin |
$93.45 |
$68.37 |
$47.64 |
$41.46 |
$36.52 |
$287.43 |
|
Atorvastatin a |
$568.17 |
$617.58 |
$670.50 |
$701.80 |
$717.63 |
$3,275.68 |
|
Rosuvastatin |
$21.31 |
$105.00 |
$202.37 |
$292.73 |
$335.19 |
$956.61 |
|
Total |
$992.36 |
$1,028.46 |
$1,091.51 |
$1,190.63 |
$1,229.37 |
$5,532.33 |
a Atorvastatin includes atorvastatin with amlodipine combination product
Recommendation and Reasons:
The PBAC considered the request for advice from the Minister for Health in relation
to the request made in the Senate on 22 November 2010 by Senator Xenophon (also representing
Senator Fielding) on any new evidence on whether or not two medicines, rosuvastatin
and atorvastatin, should be included in the existing statins therapeutic group. The
PBAC also considered the Terms of Reference for the Statins Review, which it had ratified
in August 2011.
The PBAC recalled that in July 2005 it advised the Minister and the Pharmaceutical Benefits Pricing Authority that:
- Atorvastatin was more effective than simvastatin in lowering LDL-cholesterol (LDL-C).
- The relative price differential [at the time, an average of approximately 12.5%] modelled in the submission’s cost effectiveness analysis was acceptable.
- Any further price change in simvastatin should not result in any increase in this price relativity.
- The only basis for judging whether the price relativity could be further increased would be an incremental cost effectiveness analysis based on major cardiovascular events measured directly in randomised trials rather than based on predictions modelled from surrogate outcomes.
Rosuvastatin was recommended for listing at the July 2006 PBAC meeting on a cost-minimisation basis with atorvastatin, with the rosuvastatin to atorvastatin ratio of equi-effective doses being 1:3. The PBAC considered that:
- The price of rosuvastatin should remain linked to the price of both atorvastatin and simvastatin, and that any further price change in simvastatin should not result in any increase in the price relativity of rosuvastatin to simvastatin.
- Furthermore, the only basis for judging whether the price relativity to simvastatin could be further increased would be an incremental cost effectiveness analysis based on major cardiovascular events measured directly in randomised trials rather than based on predictions modelled from surrogate outcomes.
The Statins-Higher Potency therapeutic group (atorvastatin, rosuvastatin) was formed in September 2009, as a separate group to the existing ‘Statins’ therapeutic group (simvastatin, pravastatin). On 1 April 2012, following price reductions from price disclosure for simvastatin and pravastatin, the Statins therapeutic group comprising these two F2 medicines (simvastatin and pravastatin) was abolished.
The PBAC noted the advice provided prior to this meeting by its sub-committees and correspondence from a number of clinicians. It also considered inputs from the sponsors over the course of the review. These included the original input to the review prior to the December 2011 Preliminary Report; the subsequent comments received after its release and the release of the Final Preliminary Report in February 2012; the sponsor submissions lodged in March 2012 to the July 2012 PBAC meeting; and the sponsors’ Pre-Sub-Committee and Pre-PBAC Responses including the 4 July 2012 letter from the Cholesterol Treatment Trialists’ Collaboration (CTTC) group which provided comments on the issues raised on the Review’s Report.
The PBAC discussed the systematic literature review that was undertaken to collate any new evidence of the comparative safety and effectiveness across pravastatin, simvastatin, rosuvastatin and atorvastatin. The search encompassed both primary and secondary prevention trials and was kept intentionally broader than the patient populations currently eligible for reimbursement on the PBS due to the complexity of patient eligibility on the PBS for statins. Comparative effectiveness was assessed primarily in terms of clinical outcomes, given PBAC’s stated preference to assess the comparative benefits of drugs in terms of treatment outcomes that are directly discernible and meaningful to patients.
The PBAC considered issues on the inclusion and exclusion of particular trials and accepted the ESC advice that the ASPEN trial which compared atorvastatin 10 mg daily with placebo should have also been included in the review given that at least 50% of patients in the trial would be eligible for reimbursement on the PBS. However, the PBAC considered that the overall impact of excluding the ASPEN trial from the statins review is likely to be small.
More important was the inclusion of GISSI-HF and CORONA, two large rosuvastatin trials, which confounded the size of the treatment effect of the higher-potency statins because although patients enrolled in these trials would qualify for treatment with statins under the current PBS eligibility criteria, they had advanced heart failure and so were not exchangeable with other trials considered. The size of the treatment effect of statins was likely to be different for patients with advanced heart disease versus patients with other conditions that would qualify them for treatment with statins under the current PBS eligibility criteria. Much of the effect, or rather lack of additional effect, of the higher-potency statins in comparison with simvastatin and pravastatin was driven by the CORONA and GISSI-HF trials which were not exchangeable with other trials in the indirect mixed treatment comparison (MTC).
The PBAC agreed with the ESC that the inconsistency of the results produced across the MTC is likely to be caused by the heterogeneity in the trials, in particular differences in patient populations and trial characteristics or to random variations. This is reflected in the modelled economic analysis that uses the clinical inputs from the MTC. An additional problem is the small numbers of cardiovascular events, resulting in statistically imprecise estimates of treatment effect. This is further compounded in the economic model, which takes the small differences in clinical event rates from the trials and extrapolates them over a lifetime time horizon.
However, an IPD meta-analysis, which would be methodologically superior to a MTC approach, could not be conducted during the Review to address its specific Terms of Reference; and while the CTTC individual patient data (IPD) meta-analysis has the advantage of being less subject to heterogeneity (i.e. lack of exchangeability), the randomised controlled trials included did not fully meet the Review’s specified Terms of Reference.
The PBAC noted the approach and the results of the CTTC IPD meta-analyses. They confirm that, for statins, the degree of treatment benefit in clinical terms is related to the degree of LDL-C lowering, with the relative risk of major vascular events and major coronary (RR per 1 mmol/L LDL-C reduction) being 0.76 (95% CI: 0.73-0.79). The analyses also showed that, over the ranges of baseline LDL-C and LDL-C reductions observed in the trials, this relationship was consistent in patients with and without a history of cardiovascular disease, baseline level of risk and baseline cholesterol levels.
With respect to safety, the PBACnoted that the clinical significance of the relatively small increase in risk of developing diabetes detected in the trials was not yet clear, particularly given the demonstrated reduction of cardiovascular events in patients with diabetes using statins for cholesterol lowering. There was no effect of statin treatment on newly diagnosed cancer or cancer mortality incidence.
The PBAC also acknowledged the safety concerns in relation to the 80 mg dose of simvastatin, which was an issue in clinical practice, but noted that no adverse events for any of the statins had been captured in the modelled economic analyses and rare adverse events would not have altered the outcomes of the modelled economic analyses.
The PBAC considered that the overall use of statins was likely to continue to rise steadily and the cost to Government may continue to increase for some time as the rosuvastatin portion of the market increases and until atorvastatin prices reduce further through Government pricing policies.
The PBAC concluded that although there is a large volume of research about the treatment
effect and safety of statins, there is a paucity of trials addressing the specific
questions being asked by the Terms of Reference of the review. The PBAC considered
that the problems with the exchangeability of trials eligible for inclusion on the
MTC and the small number of events in those trials may be the basis of the results
suggesting that there may be no additional benefit of the higher potency statins over
lower potency statins. On the other hand, the CTTC IPD meta-analyses of 2010 and
2012 continue to show a consistent relationship between LDL-C lowering and cardiovascular
events and overall survival in patients treated with statins. Therefore, the PBAC
reaffirmed its recommendations of 2005, 2006 and 2007 that atorvastatin and rosuvastatin
are more effective than simvastatin in lowering LDL-C, and that atorvastatin and rosuvastatin
should be treated as interchangeable on an individual patient basis.
Furthermore, the PBAC considered that the evidence available to date does not change
its previous advice to the Minister that a relative price differential of 12.5%, on
average, between atorvastatin and simvastatin was acceptable. However, the PBAC noted
that, prior to the introduction of Expanded and Accelerated Price Disclosure to simvastatin
on 1 April 2012, the price differential was about 30% above the price differential
considered by the PBAC in 2005 to be cost-effective, and has since increased further.
The PBAC reiterated its previous advice that the only basis for judging whether the
price relativity could be further increased would be an incremental cost effectiveness
analysis based on major cardiovascular events measured directly in randomised trials
rather than based on predictions modelled from surrogate outcomes. Overall, the evidence
available to date did not enable the PBAC to be satisfied that a greater price differential
than the one agreed to in 2005 would be acceptable.




