Ipilimumab, concentrate solution for I.V. infusion, 50 mg in 10mL, 200 mg in 40 mL, Yervoy® - November 2012
Public Summary Document
Product: Ipilimumab, concentrate solution for I.V. infusion, 50 mg in 10mL, 200 mg in 40 mL,
Yervoy®
Sponsor: Bristol-Myers Squibb Australia Pty Ltd
Date of PBAC Consideration: November 2012
1. Purpose of Application
The re-submission sought a Section 100 (Efficient Funding of Chemotherapy) Private Hospital Authority Required and Public Hospital/Clinic Authority Required (Streamlined) listing for treatment of patients with unresectable stage III or stage IV malignant melanoma who have not responded to or were intolerant to prior systemic therapy for metastatic disease.
2. Background
The PBAC has considered ipilimumab for malignant melanoma at two previous meetings in July 2011 and March 2012.
Refer to the July 2011 and March 2012 ipilimumab Public Summary Documents for further information.
3. Registration Status
Ipilimumab was TGA registered on 4 July 2011 as monotherapy, for the treatment of patients with unresectable or metastatic melanoma who have failed or are intolerant to prior therapy.
4. Listing Requested and PBAC’s View
Section 100 (Efficient Funding of Chemotherapy)
Private Hospital Authority Required
Public Hospital Authority Required (STREAMLINED)
Induction treatment as monotherapy of a patient with unresectable stage III or stage IV malignant melanoma who has failed or is intolerant to prior therapy for metastatic disease:
- Lack of response is defined as failure to achieve or sustain a partial or complete response or stable disease.
- Intolerance to prior systemic therapy is defined as Grade 3 or 4 toxicity that is therapy related.
Note that for patients who commence therapy with ipilimumab:
- Decisions concerning efficacy should await completion of the entire induction regimen (four doses) and should be made in conjunction with established criteria for immunological responses. However induction may be ceased or delayed if symptomatic progressive disease or intolerable adverse events occur and if, in the opinion of the clinician, continuation of treatment poses a risk to the patient.
- Tumour responses may occur beyond the initial 12 week induction phase and evaluation for potential later responses should be undertaken regularly for the first year.
- Re-induction with 4 additional doses of ipilimumab should only be commenced in patients whose disease has progressed following an initial objective response to therapy:
- Where response to therapy is defined as either:
(i) sustained stable disease of ≥ 3 months duration; or
(ii) achievement of an initial objective response (partial or complete response).
Note:
Doses greater than 3 mg per kg every 3 weeks for a total of 4 doses will not be PBS-subsidised.
The patient’s body weight must be documented in the patient’s medical records at the
time treatment is initiated.
Section 100 (Efficient Funding of Chemotherapy)
Private Hospital Authority Required
Public Hospital Authority Required (STREAMLINED)
Re-induction treatment as monotherapy of a patient with unresectable stage III or
stage IV malignant melanoma who has progressive disease after achieving an initial
response to PBS-subsidised ipilimumab as induction treatment.
Note:
Doses greater than 3 mg per kg every 3 weeks for a total of 4 doses will not be PBS-subsidised.
The patient’s body weight must be documented in the patient’s medical records at the
time.
The PBAC considered that the requested PBS restriction be developed so as to permit first-line use of ipilimumab while remaining consistent with the TGA registration. See Recommendations and Reasons.
5. Clinical Place for the Proposed Therapy
Melanomas are malignant tumours derived from melanocytes. Advanced melanoma (unresectable stage III to stage IV or metastatic melanoma) is an aggressive and invasive disease, with a median survival of approximately 6 to 9 months.
The aim of treatment in advanced melanoma is to optimally manage each stage of disease with a view to extending overall survival. Therapies for advanced melanoma are limited and include systemic therapy (dacarbazine, fotemustine or temozolomide), palliative care/radiotherapy, palliative surgery or no treatment.
The resubmission presented three treatment algorithms based on neither ipilimumab nor vemurafenib being PBS-listed, only ipilimumab being listed, and both being listed.
If ipilimumab is listed, the submission’s treatment algorithm suggested it to be a second-line therapy for patients who received chemotherapy or were part of a clinical trial in the first-line.
For PBAC’s view, see Recommendation and Reasons.
6. Comparator
The submission nominated dacarbazine (DTIC) and fotemustine as the main comparators. This is as previously accepted by the PBAC.
7. Clinical Trials
The PBAC noted that no new comparative clinical data were presented but that the resubmission presented four new pieces of information relating to the durability of ipilimumab’s effect. These were:
1) Five year follow-up from ipilimumab clinical trials CT-004 (4.5 year follow-up), CT-007, CT-008, and CT-022;
2) Four year follow-up from ipilimumab clinical trial CT-024;
3) Long-term (five years and greater) follow-up from 177 metastatic melanoma patients treated with ipilimumab. The resubmission cited two additional papers outlining existing longer-term follow-up than five years. One of the papers (Postow et al 2012) is a commentary on the other (Prieto et al. 2012);
4) An expert statement related to ipilimumab and durability.
These data did not include follow-up data for the key study CT-020 due to study closure. Overall, none of the new studies included a comparator group, preventing the more precise identification of the extent to which the plateau in survival gain occurs without ipilimumab.
Study CT-004 was a randomised phase II study for patients with advanced (i.e. unresectable Stage IIIc or IV) melanoma in both a first- and second-line setting. Patients received either 3 mg/kg or 10 mg/kg. This population differed from the proposed listing in the dose and the line of therapy. Note that only 4.5 year follow-up data was available.
Study CT-007 was a randomised phase II study for patients with previously treated, advanced (unresectable Stage IIIc or IV) melanoma received 10 mg/kg ipilimumab with or without budesonide. This study did not report the appropriate dosage for the proposed listing. Like CT-004, it was unknown what OS these patients would have experienced without ipilimumab.
Study CT-008 reported an open-label, single-arm, multicenter phase II study for patients with previously treated, advanced (unresectable Stage IIIc or IV) melanoma receiving 10 mg/kg ipilimumab. As with Study CT-007, Study CT-008 did not report the appropriate dosage for the proposed listing and did not have a comparator group.
Study CT-022 was a randomised phase II study for patients with previously treated, advanced (unresectable Stage IIIc or IV) melanoma received either 0.3 mg/kg, 3 mg/kg or 10 mg/kg ipilimumab.
Study CT-024 presented 4 year survival data and used a 10 mg/kg dose of ipilimumab alongside dacarbazine versus dacarbazine and placebo. The patient population was treatment naive. The patient population and proposed intervention were different from that proposed in the resubmission listing.
The following points about the Prieto et al. (2012) study were noted:
- The patient populations under the three protocols differed relative to the proposed listing
- With the exception of cohort 1 in protocol 1, the dosing in each of the trials was not that given in the proposed listing.
- There was no comparator group (i.e. not receiving ipilimumab), so it was difficult to ascertain whether the plateau effect is a result of the nature of the disease, or of the effect of treatment.
The resubmission also presented three pieces of new information relating to recent ‘real-world’ post-registration data. These were:
1) The most recent Periodic Safety Update Report (PSUR);
2) Italian ‘real-world’ data relating to efficacy, safety, and rates of re-induction from five abstracts submitted to the European Society for Medical Oncology (ESMO) conference in October 2012; and
3) Australian ‘real-world’ data resulting from the Patient Access Program (which existed between the 1st August 2011 and the 15th April 2012).
The table below details the published trials presented in the submission.
|
Trial ID/First Author |
Protocol title/ Publication title |
Publication citation |
|
Direct randomised trial(s) |
||
|
CT-20
Hodi FS et al. |
Improved survival with ipilimumab in patients with metastatic melanoma. |
The New England Journal of Medicine (2010); 363(8):711-23 |
|
Publications from CT-20 |
||
|
ASCO Annual Meeting Abstracts |
|
Journal of Clinical Oncology (2010); 28(18)
|
|
Haanen JB et al. |
Ipilimumab improves overall survival in patients with previously treated, advanced melanoma: Long-term survival results from a phase III trial.
|
Annals of Oncology (2010); 21: p. viii402 |
|
Hodi FS et al. |
Re-induction with ipilimumab, gp100 peptide vaccine, or a combination of both from a phase III, randomized, double-blind, multicenter study of previously treated patients with unresectable stage III or IV melanoma
|
Journal of Clinical Oncology (2010); 28(15) |
|
Lebbe C et al. |
Ipilimumab improves survival in previously treated, advanced melanoma patients with poor prognostic factors: Subgroup analyses from a phase III trial.
|
Annals of Oncology (2010); 21: p. viii401 |
|
Lorigan P et al. |
Clinical response to ipilimumab: Effect of systemic corticosteroids used to manage immune-related adverse events (irAEs).
|
Annals of Oncology (2010); 21: p. viii404 |
|
O'Day S et al. |
A phase III, randomized, double-blind, multicenter study comparing monotherapy with ipilimumab or gp100 peptide vaccine and the combination in patients with previously treated, unresectable stage III or IV melanoma
|
Journal of Clinical Oncology (2010); 28(18) |
|
Robert C et al. |
Re-induction with ipilimumab, GP100 peptide vaccine, or a combination of both in a phase III study of previously-treated patients with advanced melanoma: Update of clinical characteristics of patients.
|
Annals of Oncology (2010); 21: p. viii403-viii404 |
|
Wolchok JD et al. |
Ipilimumab efficacy and safety in patients with advanced melanoma: A retrospective analysis of HLA subtype from four trials.
|
Cancer Immunity (2010); 10(9) |
|
Long-term (five years and greater) follow-up studies |
||
|
Postow MA et al. |
The Antitumor Immunity of Ipilimumab: (T-cell) Memories to Last a Lifetime?
|
Clin.Cancer Res (2012); 18(7):1821-23 |
|
Prieto PA et al. |
CTLA-4 Blockade with Ipilimumab: Long-Term Follow-up of 177 Patients with Metastatic Melanoma. |
Clin. Cancer Res (2012); 18(7):2039-47 |
8. Results of Trials
The following table summarises the survival benefit (as presented in the previous submission) seen in Trial CT-020.
Results of Survival for CT-020 (intention-to-treat (ITT))
|
Outcomes |
IPI N=137 |
IPI +gp100 N=403 |
gp100 N=136 |
|
Median OS mths (95% CI) |
10.1 ( 8.0, 13.8) |
10.0 (8.5, 11.5) |
6.4 (5.5, 8.7) |
CI = confidence interval; N = total participants in group
The PBAC noted that the median survival in CT-020 (ITT) for the ipilimumab monotherapy arm was 10.1 months (95% CI: 8.0 months to 13.8 months), compared to 6.4 months in the gp100 peptide vaccine arm (95% CI: 5.5 months to 8.7 months). Overall, the PBAC considered that there is evidence of a plateau effect with ipilimumab treatment which provides at most up to 10% additional longer term survivors. Furthermore, there is some evidence that the effect is durable but the magnitude of the benefit remains a source of uncertainty with survival curves at the end of the time horizon driven by extremely small patient numbers. Small changes in the proportions of CT-020 patients in each disease stage will result in significant changes to the incremental benefit of treatment with ipilimumab. The PBAC acknowledged that this uncertainty was unlikely to be resolved. See Recommendation and Reasons.
Five year follow-up from ipilimumab clinical trials CT-004 (4.5 year follow-up), CT-007, CT-008, and CT-022
The percentage of patients alive after 4.5 to 5 years of ipilimumab treatment varied but were in a similar range to the percentage figures reported in Prieto et al. (2012).
Long-term (five years and greater) follow-up from 177 metastatic melanoma patients treated with ipilimumab
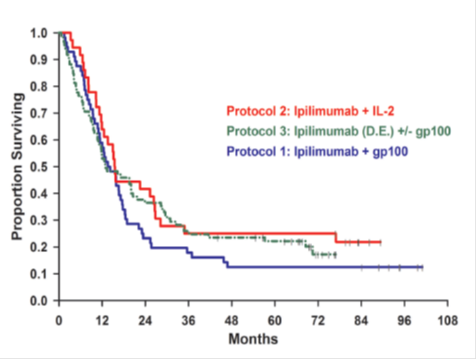
The results of the Prieto et al. (2012) study are shown in the table below.
|
|
Protocol 1 |
|
Protocol 2 |
Protocol 3 (N=88) |
|
|
|
Cohort 1 |
Cohort 2 |
Only cohort |
Cohort 1 |
Cohort 2 |
|
HLA-A*0201 status (and N) |
HLA-A*0201-positive patients (N=29) |
HLA-A*0201-positive patients (N=27) |
HLA-A*0201 not stated (N=36). |
HLA-A*0201-positive patients |
HLA-A*0201-positive patients |
|
Years of recruitment |
2002-2004 |
2002-2004 |
2003-2004 |
2004-2005 |
2004-2005 |
|
Drug and dosage |
3mg/kg ipilimumab every three weeks in conjunction with subcutaneous injections of gp100 peptides |
Initial dose of 3mg/kg ipilimumab, followed by 1mg/kg ipilimumab every three weeks in conjunction with subcutaneous injections of gp100 peptides |
Three patients per dose level received ipilimumab at 0.1, 0.3, 1, and 2 mg/kg; the last 24 patients received ipilimumab at 3 mg/kg. All patients received high-dose (720,000 IU/kg) intravenous IL-2 (given as tolerated every 8 hours up to a maximum of 15 doses) |
Ipilimumab alone starting at 3 mg/kg. After 2 cycles at 3 mg/kg, if an OR or a grade III/IV IRAE did not occur, the patient received the next 2 cycles at 5 mg/kg. If after 2 cycles at 5 mg/kg and an OR or a grade III/IV IRAE did not occur, the patient received the next 2 cycles at 9 mg/kg. HLA-A_0201–positive patients received ipilimumab in the same dose-escalating manner; however, they were also randomized to receive ipilimumab alone or in conjunction with the 2 gp100 peptides as given in protocol 1. |
|
|
Median follow-up* |
92 months |
84 months |
71 months |
||
|
Median survival* |
14 months |
16 months |
13 months |
||
|
5-year survival* |
13% |
25% |
23% |
||
OR = Objective Clinical Response; IL-2 = interleukin-2; IRAE = immune-related adverse
event
Note: * The resubmission presented median follow-up, median survival and 5-year survival
by protocol rather than by cohort
The results under the three protocols are presented diagrammatically below.
OS for all patients in Prieto et al (2012), separated by protocol
Exploration of efficacy and durability in survivors beyond 12 weeks (Landmark Analysis)
The resubmission presented a re-analysis of Study CT-020, which was called the Landmark analysis. The premise of the analysis was given in the resubmission as follows:
“A large proportion of patients in the ipilimumab arm of the key clinical trial (CT-020) were discontinued due to adverse events, progression or death before a full course of ipilimumab treatment could be administered. Consequently the results from the overall survival analysis, demonstrating a hazard ratio for survival of 0.66, are potentially biased against ipilimumab as patients who discontinued or died early in the trial did not have an opportunity to enter the responder group post the 4th ipilimumab injection.”
The resubmission therefore presented a Landmark analysis exploring the survival of those patients who remained on therapy at 12 weeks. This period reflected the time period for a full course of ipilimumab treatment in CT-020.
The PBAC did not find the Landmark analysis informative to its deliberations. See Recommendation and Reasons.
Exploration of recent ‘real-world’ post-registration data
The submission claimed that the results from the European Expanded Access Programme (EAP) in Italy supported the results seen in the other trials mentioned above.
The Australian ‘real-world’ Patient Access Program (which existed between the 1 August 2011 and the 15 April 2012) was not intended to be a trial, so survival and other outcomes were not captured. The purpose of presenting the data was to provide some information which may be of use in estimating financial implications of listing.
New toxicity data from the latest Periodic Safety Update Report (PSUR) did not identify any new safety concerns.
9. Clinical Claim
The resubmission claimed that in the treatment of unresectable or metastatic melanoma patients who have received prior therapy, ipilimumab 3 mg/kg is superior in efficacy to BSC (DTIC/fotemustine), and has a different safety profile, with irAEs (immune-related adverse events) which are manageable and controllable.
This was the same clinical claim presented in both previous submissions. This was reasonable, although the magnitude of the incremental benefit of ipilimumab remained uncertain as the resubmission was reliant on extrapolation of trial results to a ten-year time horizon. The PBAC previously noted that some studies show less than 10% are alive at 10 years (Balch et al. (2009)).
10. Economic Analysis
An updated modelled economic evaluation (cost-utility analysis/cost-effectiveness analysis) based on the claim of superior efficacy was presented which was consistent in structure and inputs with that presented in the March 2012 submission. The only change was a decrease in price. The PBAC considered that the selection of the point at which the Kaplan Meier hazard function changes (i.e. the start of the plateau section) for both the ipilimumab and control arms should have been explored in a sensitivity analysis. The PBAC considered that the plateau effect extrapolated from the Kaplan Meier hazard function was based on small numbers of patients. At 28 months only 18 and 7 patients remain in the ipilimumab and gp100 arms respectively. The PBAC further considered that these patient numbers may be too small to allow a more reliable extrapolation to 5 and 10 years. See Recommendation and Reasons.
The submission presents an ICER in the range of $45,000 - $75,000/QALY based on overall survival, extrapolated to 10 years (from 2 years in the trial CT-020) and applying EQ5D values derived from within trial data.
The PBAC considered the base case ICER to be high and uncertain. The PBAC noted that the extrapolation method and point at which extrapolation occurs had only minimal impact on the ICER. Sensitivity analyses show that the model was sensitive to a time horizon of 5 years (an ICER in the range of $105,000 - $200,000 QALY/gained) and the choice of utility weights (application of SF-6D weights increased the ICER to be in a range of $75,000 - $105,000/QALY gained). See Recommendation and Reasons.
11. Estimated PBS Usage and Financial Implications
The likely number of patients per year was estimated in the submission to be significantly less than 10,000 in Year 5
The submission’s estimated net cost per year to the Government was in the range of $60 - $100 million in Year 5.
The PBAC considered that the utilisation and financial estimates for ipilimumab require amendments from what was presented by the sponsor in the submission. See Recommendation and Reasons.
12. Recommendation and Reasons
The PBAC recommended ipilimumab for monotherapy in a patient with unresectable Stage III or Stage IV malignant melanoma in the context of high-clinical need and no effective therapies available. The PBAC again acknowledged the high clinical need for effective drugs to treat malignant melanoma.
The PBAC noted the proposed treatment algorithm placed ipilimumab as a second line therapy after failure of BRAF inhibitors (for patients with BRAF mutant tumours), systemic chemotherapy (for patients with BRAF wild tumours) or experimental treatments (for clinical trial participants). The PBAC considered that the submission had overestimated the opportunities for first line treatment in the context of a clinical trial. The PBAC noted that systemic chemotherapy with dacarbazine (DTIC) or fotemustine has minimal efficacy and significant toxicity and that the treatment options for melanoma patients have rapidly evolved since trial CT-020 was undertaken.
Further, the PBAC noted that the sponsor’s expert advisory panel considered a requirement to use DTIC or fotemustine to be contrary to clinical judgment and would therefore be unlikely to be observed in practice. This appeared to be inconsistent with the advisory board’s view that utilisation of DTIC would increase as a direct result of the listing of ipilimumab. The PBAC noted that DTIC is not PBS subsidised for this indication whereas fotemustine is PBS subsidised
Therefore, the PBAC concluded that a requirement for patients to first try then fail ineffective and toxic first-line chemotherapy would not be clinically appropriate and requested that the PBS restriction be developed so as to permit first-line use of ipilimumab.
The PBAC noted that no new comparative clinical data were presented, but the submission provided four new pieces of information related to the durability of the clinical effect of ipilimumab beyond five years. These are: (1) five year follow-up from ipilimumab clinical trials CT-004 (4.5 year follow-up), CT-007, CT-008, and CT-022; (2) four year follow-up from ipilimumab clinical trial CT-024; (3) long-term (five years and greater) follow-up from 177 metastatic melanoma patients treated with ipilimumab; and (4) an expert statement related to ipilimumab and durability. These data did not include follow-up data for the key study CT-020 due to study closure. Overall, none of the new studies included a comparator group, preventing the more precise identification of the extent to which the plateau in survival gain occurs without ipilimumab.
The PBAC noted that the median survival in CT-020 (ITT) for the ipilimumab monotherapy arm was 10.1 months (95% CI: 8.0 months to 13.8 months), compared to 6.4 months in the gp100 peptide vaccine arm (95% CI: 5.5 months to 8.7 months). Overall, the PBAC considered that there is evidence of a plateau effect with ipilimumab treatment which provides at most up to 10% additional longer term survivors. Furthermore, there is some evidence that the effect is durable but the magnitude of the benefit remains a source of uncertainty with survival curves at the end of the time horizon driven by extremely small patient numbers. Small changes in the proportions of CT-020 patients in each disease stage will result in significant changes to the incremental benefit of treatment with ipilimumab. The PBAC acknowledged that this uncertainty was unlikely to be resolved.
The PBAC noted that the submission presented a modelled economic evaluation which was consistent in structure and inputs with that presented in the March 2012 submission. The only change was a decrease in price. The PBAC considered that the selection of the point at which the Kaplan Meier hazard function changes (i.e. the start of the plateau section) for both the ipilimumab and control arms should have been explored in a sensitivity analysis. The ESC considered that the plateau effect extrapolated from the Kaplan Meier hazard function was based on small numbers of patients. At 28 months only 18 and 7 patients remain in the ipilimumab and gp100 arms respectively. The PBAC considered that these patient numbers may be too small to allow a more reliable extrapolation to 5 and 10 years.
The PBAC noted that the submission presented a Landmark analysis which excluded patients who did not complete 12 weeks of therapy, in order to clarify the magnitude of treatment effect in responders. The PBAC did not find the Landmark analysis informative to its deliberations.
Therefore, the PBAC considered that the base case ICER in the range of $45,000 - $75,000/QALY gained is high and uncertain. The PBAC noted that the extrapolation method and point at which extrapolation occurs had only minimal impact on the ICER. Sensitivity analyses show that the model is sensitive to a time horizon of 5 years (an ICER in the range of $105,000 - $200,000/QALY gained) and the choice of utility weights (application of SF-6D weights increased the ICER to be in a range of $75,000 - $105,000).
The PBAC considered that the utilisation and financial estimates for ipilimumab require amendments from what was presented by the sponsor in the submission. The PBAC noted that the base case now assumed the number of patients treated with ipilimumab per year to be a figure less than 10,000. The total PBS financial impact estimated by the sponsor increased to greater than $100 million over the first 5 years by more than $100 million compared to the previous submission. The PBAC noted that this increase was driven by a substantially higher proportion of eligible patients, given the updated clinical algorithm. However, with the estimated patients diagnosed with unresectable Stage IIIC/IV melanoma each year, the possible number of patients eligible for ipilimumab is likely to be substantially higher.
The PBAC noted that treatment outside the restriction in a first-line setting was associated with significant financial risk both in terms of increased patient numbers and the potential for use of 10 mg/kg doses, compared with 3 mg/kg doses in a second-line setting.
The PBAC noted the high unmet clinical need for treatments for metastatic melanoma with proven survival advantage. The PBAC considered that the proposed ICER in the range of $45,000 - $75,000 per QALY was high but acceptable if the modelled survival gain is observed in clinical practice. The PBAC noted that the ICER is highly dependent on the duration of survival.
The PBAC, although concerned about the cost-effectiveness of ipilimumab if the claimed survival gain were not observed in practice, recommended the listing of ipilimumab for metastatic melanoma, subject to risk-share arrangements involving the following aspects:
- Appropriate use
- Development of a PBS restriction aligned with the TGA approved indication but that does not necessitate exposure to cytotoxic chemotherapy. Given the possible complexity of such a restriction, this will be finalised at a later date. In effect, this allows the subsided use of ipilimumab as first-line treatment at a dose of 3 mg/kg.
- Maintaining cost-effectiveness
- Implementation of a mechanism to verify the anticipated overall survival benefits of ipilimumab in real world clinical practice in Australia. This approach should be designed in such a way that it provides evidence of whether or not the extent of the survival benefit modelled in the submission and which was used in the calculation of cost-effectiveness, was realised in Australian clinical practice. The sponsor would be expected to rebate the cost of difference in performance between observed versus predicted benefits of ipilimumab. As the expected survival proportion at 2 years is higher in the first-line setting, further information is required from the sponsor in order to construct any risk-share arrangements. The PBAC requested that the details of this pay for performance arrangement be provided for its approval prior to incorporation in a deed of agreement between the sponsor and the Commonwealth.
- Managing financial risk
- Negotiation of a suitable risk share agreement with significant rebates in order to manage the risks to Commonwealth financial expenditure in terms of number of patients and dose
In making this recommendation the PBAC noted the consumer comments on this item.
Recommendation:
IPILIMUMAB, concentrate solution for I.V. infusion, 50 mg in 10 mL, 200 mg in 40 mL
Restriction: SECTION 100 (EFFICIENT FUNDING OF CHEMOTHERAPY)
Private Hospital Authority Required
Public Hospital Authority Required (STREAMLINED)
To be finalised
13. Context for Decision
The PBAC helps decide whether and, if so, how medicines should be subsidised in Australia. It considers submissions in this context. A PBAC decision not to recommend listing or not to recommend changing a listing does not represent a final PBAC view about the merits of the medicine. A company can resubmit to the PBAC or seek independent review of the PBAC decision.
14. Sponsor’s Comment
Bristol-Myers Squibb welcomes the decision by the PBAC and will work with the Government to ensure listing of ipilimumab for treatment of patients with unresectable stage III or stage IV malignant melanoma in a timely manner.




