DUSC Review on the Utilisation of Antipsychotics - August 2013
Public Summary Document
Product: Various Antipsychotics
Sponsor: Various
Date of PBAC Consideration: August 2013
1. Purpose of Item
To inform the PBAC of the main findings of a stage 2 utilisation analysis of antipsychotics by the Drug Utilisation Sub-Committee (DUSC) conducted in June 2013.
2. Background
A previous DUSC analysis of the utilisation of PBS listed antipsychotic medicines (February 2013) showed high growth in the volume of antipsychotic prescriptions, particularly newer listed products. The analysis demonstrated that there is high and inappropriate utilisation of antipsychotics in the elderly.
The prescription data also indicated some use of antipsychotics in very young patients and possible use of antipsychotics for non-PBS subsidised indications in middle-aged adults. The PBAC supported the DUSC request to further investigate the use of antipsychotics to be better informed of the utilsation of these medicines in practice.
3. The DUSC Stage 2 Utilisation Analysis
Scope
Assessment of sequential or co-administered psychotropic drugs was limited to antipsychotics and antidepressants. Other classes of psychotropic drugs, such as benzodiazepines, were beyond the scope of the stage 2 analysis. Lithium is classified as an antidepressant in the PBS Schedule and was therefore grouped with antidepressants in this report. It was noted from the DUSC report that the World Health Organisation Anatomical Therapeutic Chemical (ATC) classifies lithium as an antipsychotic.
In addition to antipsychotics and antidepressants, anti-dementia drugs (anticholinesterases and memantine) were also analysed as part of medicine regimens in the elderly.
General method
The prescription data was extracted from the Medicare Department of Human Services (DHS) Pharmacy Claims database (DHS-Medicare is the Government agency that collects information on all PBS prescriptions dispensed in Australia).
Due to limitations in computation capacity, analyses within very large data sets (for example, all prevalent patients) were reduced to a 10% patient random sample. Where small numbers of patients over time were analysed (for example initiating patients), a 100% sample was used.
The extracted data included:
- Patient’s age at either date of first prescription supply (initiation base extract) or at 31 October 2012 (prevalence analyses).
- Patient’s gender.
- Prescriber type. (Identification of specialty used a major specialty list maintained by
- DHS. This classification depends on both the prescribers’ qualifications and Medicare billing practice).
All antipsychotics (ATC N05A) listed on the PBS and RPBS in Australia were included in the analysis except clozapine, since no de-identified patient information such as gender or age is provided when clozapine is supplied through public hospitals.
4. Results of the utilisation analysis
The PBAC were asked to note DUSC’s stage 2 findings:
- that there was inappropriate use of antipsychotics in adult patients aged 25-59 years, for non-subsidised and off-label indications. This suggested several quality use of medicines issues including the potential for side effects;
- off-label and non-subsidised use of antipsychotics was most evident with the use of the 25mg strength of quetiapine. The DUSC analysis estimated that of all patients on a regimen that contained a PBS listed antipsychotic, 38% of these included quetiapine (see Figure 1);
- of the patients aged 20-59 years old who were prescribed quetiapine, 13.8% were taking an antidepressant + quetiapine 25mg, and 9.4% were taking quetiapine 25mg alone. Overall, 23.2% of patients taking quetiapine were taking only the 25mg strength;
- quetiapine 25mg is not a therapeutic dose for any of the PBS listed indications. DUSC considered that the only role for the 25mg strength of quetiapine, within the PBS restrictions, could be for dose titration in older people with bipolar disease or schizophrenia;
- that the prescriber type analysis showed more prescriptions were written by GPs compared to other health providers, but this varied by drug. Risperidone, olanzapine and quetiapine were more likely to be written and continued by GPs (see Figure 2). The DUSC noted that this may be in shared care with psychiatrists or in consultation with clinical psychologists, and patients may also be treated in hospital. The large proportion of first original prescriptions written by GPs (approximately 66%) suggested the use of these medicines may be for the treatment of conditions other than schizophrenia or bipolar disorder;
- that a number of initiatives were underway to support the appropriate prescribing of antipsychotic medicines for patients with dementia in residential aged care facilities;
- some of the issues of inappropriate use were related to prescribing behaviour, and it may help to involve the respective Royal Australian Colleges in educating prescribers in both general practice and hospital settings.
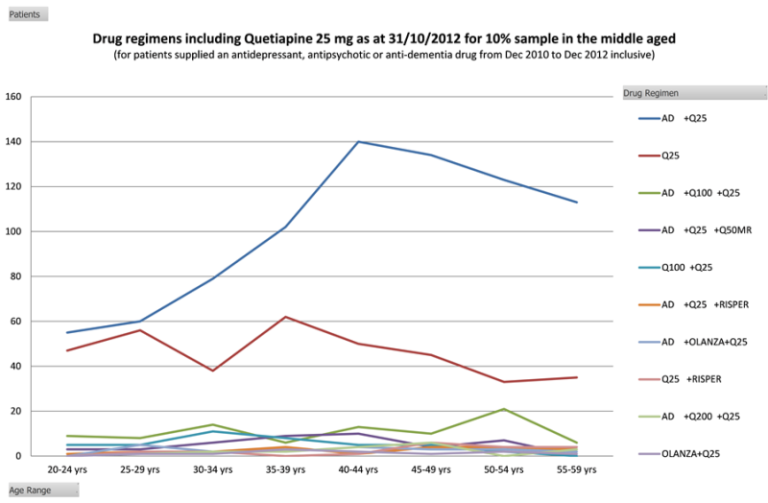
Figure 1: Top 10 drug regimens that include quetiapine 25mg
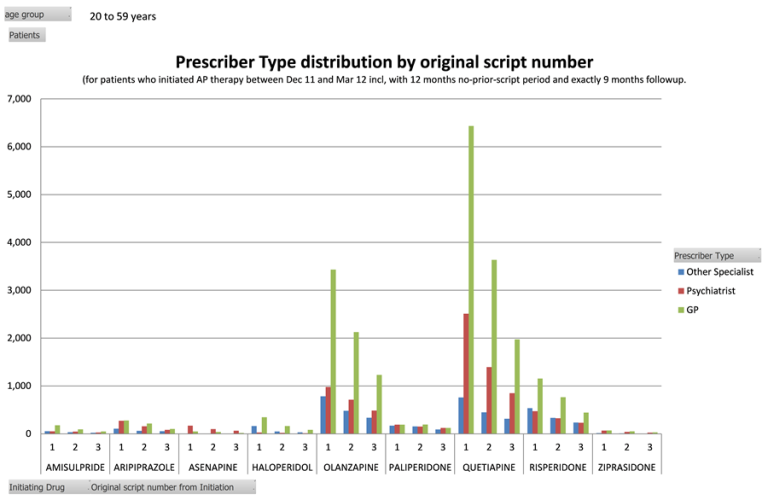
Figure 2: Original prescriptions dispensed by approved prescriber type
Y-axis is the number of original prescriptions
5. PBAC discussion
The PBAC recalled that at its April 2013 meeting, the Committee considered that changing the listings for all atypical antipsychotics to telephone Authority Required would constitute considerable inconvenience for prescribers. Moreover, the PBAC noted that prior to implementation of streamlining in 2007, there was already evidence of use of atypical antipsychotics in the elderly. Therefore the PBAC considered that it was unlikely that requiring a telephone authority would reduce overall inappropriate use of antipsychotics.
The PBAC also considered that if only selected medicines require telephone approval, utilisation may inappropriately shift to medicines with streamlined listings, or to antipsychotics with unrestricted listings, and decided that changing the Streamlined Authority listings of atypical antipsychotics to telephone authorities may not be appropriate at the time.
The PBAC considered that quetiapine 25mg formulation is not a therapeutic dose for any of the PBS listed indications and agreed with DUSC that the only role for the 25mg strength of quetiapine, within the PBS restrictions, could be for dose titration in older people with bipolar disease or schizophrenia.
The PBAC acknowledged suggestions from a sponsor of quetiapine that the PBAC consider transitioning the 25mg strength of quetiapine from Authority Required (Streamlined) to a full Authority Required listing, and that PBAC may wish to reduce the number of repeats for this presentation.
The PBAC considered that if telephone authority is required for only the 25mg strength of quetiapine, the prescribing could inappropriately shift to higher strengths or alternative agents. However given the high rate of potentially inappropriate use of antipsychotics across all adult age groups, the PBAC requested that the Department explore the potential impacts of changing the listings from streamlined authority to Authority required before finalising a recommendation.
The PBAC also considered that a smaller pack size of 25mg quetiapine would be more consistent with the place of this strength in dose titration. Therefore, the PBAC requested the Department liaise with the Therapeutic Goods Administration (TGA) and the sponsors to discuss reducing the current pack sizes of the 25mg quetiapine, from 60 tablets to 30 tablets.
The PBAC requested that the number of repeats of 25mg quetiapine be reduced from five to zero. This listing was considered to be sufficient for dose titration in bipolar disease and schizophrenia, and would encourage regular prescriber review for patients treated for non-subsidised indications.
The PBAC also referred the matter to the NPS MedicineWise to address the issues of the potentially inappropriate use, and prescribing of low dose quetiapine in adults. The PBAC considered that it was important to consult with prescribers in both general and hospital practices in the development of any proposed campaigns to better educate and promote prescriber behavioural change towards antipsychotics. The PBAC also recommended that these concerns be communicated to the Australian Medical Association and the Royal Australian and New Zealand College of Psychiatrists.
The PBAC requested that the Department explore opportunities for the DUSC utilisation analysis report to be put into the public domain, either on the Department’s website and/or in a peer reviewed medical journal.
The PBAC also recommended that DUSC review the whole class of antipsychotic drugs, 24 months after any changes in the pack size or number of repeats.
6. Recommendation
Authority Required (STREAMLINED)
|
Name, Restriction, Manner of administration and form |
Max. Qty |
No. of Rpts |
Proprietary Name and Manufacturer |
|
|---|---|---|---|---|
|
QUETIAPINE Quetiapine 25mg tablets, 60
|
1
|
0
|
APO-Quetiapine Chem mart Quetiapine Delucon 25 Pharmacy Choice Quetiapine Quetiaccord Quetiapine Actavis 25 Quetiapine-DRLA Quetiapine-GA Quetiapine GH 25 Quetiapine RBX Quetiapine Sandoz Quipine Sequase Seronia 25 Seroquel Syquet Terry White Chemist Quetiapine |
TX CH DO RI UA TA RZ GN GQ RA SZ VN PM QA AP AF TW |
|
Condition: |
Schizophrenia |
|---|---|
|
Restriction: |
Authority required (Streamlined) |
|
Clinical criteria: |
The treatment must be for dose titration purposes |
|
Administrative Advice
|
Note No increase in the maximum quantity or number of units may be authorised |
|
Administrative Advice
|
Note No increase in the maximum number of repeats may be authorised. |
|
Administrative Advice
|
Note Shared Care Model For prescribing by nurse practitioners where care of a patient is shared between a nurse practitioner and medical practitioner in a formalised arrangement with an agreed management plan. Further information can be found in the Explanatory Notes for Nurse Practitioners |
|
Condition: |
Acute mania |
|---|---|
|
Restriction: |
Authority required (Streamlined) |
|
Clinical criteria:
|
The condition must be associated with bipolar 1 disorder AND The treatment must be as monotherapy AND The treatment must be for dose titration purposes |
|
Administrative Advice
|
Note No increase in the maximum quantity or number of units may be authorised |
|
Administrative Advice
|
Note No increase in the maximum number of repeats may be authorised. |
|
Administrative Advice
|
Note Shared Care Model For prescribing by nurse practitioners where care of a patient is shared between a nurse practitioner and medical practitioner in a formalised arrangement with an agreed management plan. Further information can be found in the Explanatory Notes for Nurse Practitioners |
|
Condition: |
Bipolar 1 disorder |
|---|---|
|
Restriction: |
Authority required (Streamlined) |
|
Clinical criteria:
|
The treatment must be for maintenance treatment of bipolar 1 disorder AND The treatment must be for dose titration purposes |
|
Administrative Advice
|
Note No increase in the maximum quantity or number of units may be authorised |
|
Administrative Advice
|
Note No increase in the maximum number of repeats may be authorised. |
|
Administrative Advice
|
Note Shared Care Model For prescribing by nurse practitioners where care of a patient is shared between a nurse practitioner and medical practitioner in a formalised arrangement with an agreed management plan. Further information can be found in the Explanatory Notes for Nurse Practitioners |
7. Context for Decision
The PBAC helps decide whether and, if so, how medicines should be subsidised in Australia. It considers submissions in this context. A PBAC decision not to recommend listing or not to recommend changing a listing does not represent a final PBAC view about the merits of the medicine. A company can resubmit to the PBAC or seek independent review of the PBAC decision.
8. Sponsor’s Comment
AstraZeneca does not promote or condone any use of quetipine fumarate which is not consistent with the registered or approved indications.




