Multicomponent Meningococcal Group B Vaccine, 0.5mL, injection, prefilled syringe, Bexsero® - November 2013
Public Summary Document
Product: Multicomponent Meningococcal Group B Vaccine, 0.5mL, injection, prefilled syringe,
Bexsero®.
Sponsor: Novartis Vaccines and Diagnostics Pty Ltd.
Date of PBAC Consideration: November 2013
1. Purpose of Application
To request the inclusion of 4-component meningococcal group B vaccine (4CMenB) in the National Immunisation Program (NIP) Schedule for prevention of meningococcal B disease in infants and adolescents.
2. Background
This was the first submission to the PBAC for inclusion of 4CMenB in the NIP.
3. Registration Status
4CMenB was TGA registered on 14 August 2013, and is indicated for active immunisation against disease caused by Neisseria meningitidis group B strains. It is indicated for vaccination of individuals from 2 months of age and older.
4. Listing Requested and PBAC’s View
The submission requested inclusion of 4CMenB in the NIP with a proposed vaccination schedule as a routine 3+1 schedule in infants, a 2 dose course in adolescents and a catch-up program in older infants, toddlers and adolescents. .
The requested NIP listing is consistent with the TGA-approved indication.
5. Clinical Place for the Proposed Therapy
Invasive meningococcal B disease (IMD) is a rare disease caused by the bacterium Neisseria meningitidis. There were 184 cases (0.82 per 100,000) and approximately 11 deaths in 2011. Data collected between 1991 and 2011 indicates that incidence is bimodal, with 20% of confirmed cases aged <1 year, 22% aged 1-4 years, and the next peak in later teens, with 17% aged 15-19 years and 9% aged 20 to 24 years. Applying this pattern, around 5 of the deaths in 2011 were in those aged <5 years. IMD can also cause meningitis and sepsis, leading to long-term sequelae including: limb amputation, hearing loss, seizures, renal insufficiency, significant neurological deficits and skin scarring. In contrast to the low incidence of IMD, asymptomatic carriage of meningococci (all serotypes) is common: from 4,500 per 100,000 in infants to a peak of 23,000 per 100,000 in 19-year olds.
The vaccine contains four antigenic components, adsorbed on aluminium hydroxide: factor H binding protein (fHbp), neisserial adhesin A (NadA), neisseria heparin binding antigen (NHBA) and outer membrane vesicles (OMV) from a New Zealand epidemic strain (NZ98/254), which provides PorA P1.4. 4CMenB is intended to stimulate the production of bactericidal antibodies that recognise these antigens, and protect against a broad range of disease-causing meningococcal group B strains.
The PBAC considered that the vaccine was an important advance in vaccinology, using new technology to address issues specific to meningococcal B. While there are conjugate based and polysaccharide capsule-based vaccines currently available for other meningococcal groups, similar approaches for meningococcal B vaccines have failed because the meningococcal B polysaccharide capsule is poorly immunogenic. 4CMenB was created using an innovative approach to vaccine development known as reverse vaccinology. This process employs genomic mining to identify multiple surface-exposed antigens that are important virulence factors and that are believed to be highly conserved across most isolates.
The PBAC noted the Australian Technical Advisory Group on Immunisation (ATAGI) post-submission advice that considered although there may be clinical value in a targeted program for indigenous children aged <5 years, and patients with complement deficiency and asplenia, based on increased risk of IMD , there are significant implementation issues to consider in the delivery of programs that target specific sub-populations. In particular, that coverage is difficult to predict, and has historically been low for other targeted vaccine programs. The submission did not consider the clinical benefit or cost-effectiveness of targeting the vaccine to any high risk population.
6. Comparator
The submission nominated no vaccination as the comparator.
The PBAC agreed that the nominated comparator was appropriate, given that no vaccine against meningococcal B infection is currently available.
7. Clinical Trials
The submission presented separate studies to support vaccine efficacy in infants and in adolescents. All of the studies presented in the submission use a surrogate marker of vaccine efficacy (namely titres of ≥1:4 derived from a human serum bactericidal assay (hSBA)), historically accepted for establishing protection from meningococcal disease. No clinical outcome studies were conducted. The PBAC recalled that it had accepted the use of SBA titres, as a surrogate outcome for clinical efficacy, in its consideration of the combination vaccine for Haemophilus influenzae (Hib) and Neisseria meningitis serogroup C (MenC) compared to giving the two component vaccines concomitantly. The PBAC noted that the threshold titre value was determined based on studies from the 1960s assessing the bactericidal activity of an Army recruit population for susceptibility to group C meningococcal disease. The PBAC also noted that all previous meningococcal vaccines validated with effectiveness outcomes using this surrogate have been based upon capsular polysaccharide antigens, with or without glycoconjugation. The PBAC noted that ATAGI agreed that the use of an hSBA titre threshold of ≥1:4 is a suitable surrogate for inference of the clinical efficacy of the 4CMenB vaccine in clinical trials.
Infants: The submission presented two randomised trials comparing 4CMenB to placebo (Studies V72P12 and V72P13), in infants aged 2 months at entry, receiving 3 doses. A meta-analysis of these trials is also presented in support of the clinical claim for vaccination in infants. The submission presented additional evidence from three extension studies. Study V72P12 had one extension phase (E1) and Study V72P13 had two extension phases (E1 and E2). Evidence from Studies V72P12E1 and V72P13E1 is presented in support of the clinical claim for a booster dose at 12 months of age.
The PBAC noted that these studies were carried out with the routine concomitant vaccinations of Infanrix Hexa, Prevenar 7 and MMRV, but not rotavirus or Prevenar 13 which are currently on the NIP for infants. There is no information on potential interaction of the 4CMenB with rotavirus or some pneumococcal serotypes contained in Prevenar 13 in terms of protective immune response or potential harms.
A supportive trial, Study V72P16, investigated the impact of prophylactic paracetamol following concomitant administration of 4CMenB with the routine vaccines in infants at 2, 3 and 4 months of age.
Adolescents: The submission presented one randomised trial comparing 4CMenB to placebo (Study V72P10), in adolescents 11 to 17 years of age, receiving 1, 2 or 3 doses. The submission presented additional evidence from one extension study, V72P10E1 to demonstrate persistence of effect.
Carriage: The submission presented one randomised trial that assessed the effect of 4CMenB (2 doses) on the reduction in meningococcal B nasopharyngeal carriage in university students aged 18-24 years in the UK (Study V72_29).
Details of the trials and associated reports presented in the submission are presented in the table below.
Trials and associated reports presented in the submission
|
Trial ID |
Protocol title/ Publication title |
Publication citation |
|---|---|---|
|
Infant Studies |
||
| V72P12 |
Gossger N, Snape MD, Finn A et al. Immunogenicity of an investigational multicomponent meningococcal serogroup B vaccine (4CMenB) administered with or without routine infant vaccinations in different schedules. |
Poster presented at 29th ESPID Meeting, 7-11 June 2011, The Hague, The Netherlands. |
|
Gossger, N., Snape, M. D., et al. (2012). "Immunogenicity and tolerability of recombinant serogroup B meningococcal vaccine administered with or without routine infant vaccinations according to different immunization schedules: A randomized controlled trial.". |
JAMA - Journal of the American Medical Association 307(6): 573-582 |
|
|
Cohn, A. C. and Messonnier, N. E. (2012). "Inching toward a serogroup B meningococcal vaccine for infants." |
JAMA - Journal of the American Medical Association 307(6): 614-615. |
|
|
Vesikari, T., S. Esposito, et al. (2013). "Immunogenicity and safety of an investigational multicomponent, recombinant, meningococcal serogroup B vaccine (4CMenB) administered concomitantly with routine infant and child vaccinations: Results of two randomised trials." |
The Lancet 381(9869): 825-835. |
|
|
|
Beeretz I, Snape MD, Finn A et al. Reactogenicity and safety of multicomponent meningococcal serogroup B vaccine (4CMenB) administered with or without routine infant vaccinations in different schedules. |
Poster No. 1187. Presented at 29th ESPID Meeting, 7-11 June 2011, The Hague, The Netherlands. |
|
V72P12E1 |
Snape, MD, Finn A, Heath et al. Persistence to 12, 18 and 24 months of bactericidal antibodies induced by infant immunisation with a serogroup B meningococcal vaccine. |
Poster presented at 31st ESPID Meeting, May 28-June 1 2013, Milan, Italy. |
| V72P13 |
Vesikari, T., Esposito, S., et al. (2010). "Immunogenicity of an investigational multicomponent meningococcal serogroup B vaccine in healthy infants at 2, 4 and 6 months of age.". |
Canadian Journal of Infectious Diseases and Medical Microbiology 21(4): 183 |
|
Vesikari, T., Esposito, S., et al. (2011). "Use of an investigational multicomponent meningococcal serogroup B vaccine (4cmenb) in a clinical trial in 3630 infants." |
Archives of Disease in Childhood 96: A3. |
|
|
|
Vesikari, T., S. Esposito, et al. (2013). "Immunogenicity and safety of an investigational multicomponent, recombinant, meningococcal serogroup B vaccine (4CMenB) administered concomitantly with routine infant and child vaccinations: Results of two randomised trials." |
The Lancet 381(9869): 825-835.
|
| V72P13E1 |
Vesikari T, Prymula R, Liese J et al. Booster dose at 12 months of an investigational meningococcal serogroup B vaccine (4CMenB) in healthy toddlers previously primed at 2,4,6 months. |
Poster presented at 29th ESPID Meeting, 7-11 June 2011, The Hague, The Netherlands. |
|
Prymula R, Vesikari T, Esposito S, et al. Catch-up vaccination of healthy toddlers with an investigational meningococcal serogroup B vaccine (4CMenB) - exploration of a two-dose schedule. |
Poster presented at 29th ESPID Meeting, 7-11 June 2011, The Hague, The Netherlands. |
|
|
Toneatto D, Prymula R, Merrall E et al. Immunogenicity and reactogenicity of two-dose vaccination with investigational meningococcal b recombinant vaccine at 24 and 26 months of age. |
Poster presented at 31st ESPID Meeting, May 28-June 1 2013, Milan, Italy. |
|
|
Adolescent Studies |
||
| V72P10 |
Santolaya, M. E., O'Ryan, M. L., et al. (2012). "Immunogenicity and tolerability of a multicomponent meningococcal serogroup B (4CMenB) vaccine in healthy adolescents in Chile: A phase 2b/3 randomised, observer-blind, placebo-controlled study." |
The Lancet 379(9816): 617-624. |
|
|
Stephens, D. S. (2012). "Prevention of serogroup B meningococcal disease." |
The Lancet 379(9816): 592-594. |
| V72P10E1 |
Santolaya ME, O’Ryan MO, Valenzuela MT et al. Persistence of antibodies 18–24 months after adolescent immunization with 1–3 doses of a multicomponent meningococcal serogroup B vaccine. |
Poster presented at 31st ESPID Meeting, May 28-June 1 2013, Milan, Italy. |
|
|
Santolaya ME, O’Ryan M, Dull, P. et. al. Persistence of antibodies in adolescents 18–24 mo after immunization with one, two or three doses of 4CMenB meningococcal serogroup B vaccine. |
Human Vaccines & Immuno. Vol 9 Issue 11 June 2013 (e-pub ahead of print) http://www.landesbioscience.com/journals/vaccines/article/25505/ |
8. Results of Trials
The hSBA titre was measured in the sera of vaccinees against indicator bacterial strains, corresponding to antigens in the vaccine. Since the completion of the study, the sponsor identified a candidate serogroup B indicator strain (M10713) for measuring antigen-specific hSBA responses to the NHBA antigen. Post-hoc analyses were conducted on a small number of sera from the trials.
| Indicator strain | Antigen component of 4CMenB |
|---|---|
| 44/76 | fHbp |
| 5/99 | NadA |
| NZ98/254 | PorA P1.4 |
| M10713 | NHBA |
Infants – Primary vaccination schedule:
The submission presented a meta-analysis of studies V72P12 and V72P13 of the primary endpoint (percentage of participants with hSBA ≥1:5).
The PBAC noted that the primary objective was met for both studies against the three antigen strains in the pre-specified analysis (fHbp, NadA and PorA P1.4). The proportion of vaccinated infants achieving a hSBA ≥1:5 against antigen PorA P1.4 was less than for antigens fHbp and NadA. The PBAC noted the ATAGI observation (pre-PBAC Submission Advice, June 2013) that the proportion of participants with hSBA ≥1:5 against antigen PorA P1.4 is ‘lesser, but still acceptably high’. Post-hoc analyses were conducted in trials V72P12 (N= 36-39) and V72P13 (N= 100) to assess hSBA response against antigen NHBA. Using the lower limit of the 95% CI for the % of subjects with hSBA titer >1:5 of ≥70% to determine sufficient immune response, results from Study V72P13 met the criteria for antigen NHBA. Post-hoc analysis of participants from Study V72P12 did not meet the criteria for sufficient immune response as that the response 1 month after the 3rd immunisation was similar to baseline levels.
Infants – Booster dose:
Results for the primary endpoint (percentage of participants with hSBA ≥1:5) at one month after the third vaccination, including the results of post-hoc analyses conducted against antigen NHBA amongst 100 participants in Trial V72P13 and V72P13E1 are presented in the table below.
Number of participants with bactericidal titres (hSBA) ≥1:5, Study V72P13 and V72P13E1: PP, MITT for M10713 (NHBA)
|
Study |
Strain |
44/76 (fHbp) |
5/99 (NadA) |
NZ98/254 (PorA P1.4) |
M10713 d (NHBA) |
|---|---|---|---|---|---|
|
V72P13 4CMenB All (246)a |
Baseline n (%) 95% CI |
35 (3%) (2-4) N=1,156 |
45 (4%) (3-5) N=1,154 |
14 (1%) (1-2) N=1,160 |
33 (33%) (24-43) N=100 |
|
1 month after 3rd dose |
1,146 (100%) (99-100) N=1,149 |
1,149 (100%) (99-100) N=1,152 |
965 (84%) (82-86) N=1,152 |
84 (84%) (75-91) N=100 |
|
|
V72P13E1 Men246b |
6 months after 3rd dose (prebooster) |
348 (82%) (78-85) N=426 |
418 (99%) (97-100) N=423 |
93 (22%) (18-26) N=426 |
61 (61%) (51-71) N=100 |
|
1 month after booster |
422 (100%) (99-100) N=422 |
421 (100%) (99-100) N=421 |
404 (95%) (93-97) N=424 |
98 (98%) (93-100) N=100 |
CI: confidence interval; MITT: modified intention to treat; N: total number of participants; PP: per protocol population. a 4CMenB All (246), combined lots of 4CMenB administered at 2, 4 and 6 months. b 12B12M (1a) + 12B13M (1b) combined: in the open-label (immunogenicity) subset of V72P13, these participants had received 4CMenB + routine vaccinations at 2, 4 and 6 months of age. In V72P13E1, these participants received a 4CMenB booster and MMRV at 12 or 13 months of age. d Since the completion of the study, Novartis has identified a candidate serogroup B indicator strain (M10713) for measuring antigen-specific hSBA responses to the NHBA antigen (post-hoc immunogenicity endpoint)(MITT results).
The results observed in V72P13/E1 reflected the comparative benefits of vaccination of infants. The PBAC noted that similar results were observed in V72P12E1. Significant variation of bacterial antibody response was observed across antigens at each time point tested. The persistence of bacterial antibody 6 months after the 3rd dose and 12 months post-booster varied across the antigens. There is a considerable decline in the proportion of vaccine recipients with bactericidal antibody titres, particularly against antigens PorA P1.4 and NHBA.
4CMenB given at 2, 4, 6, and 12 months (percentage of participants with bactericidal titres (hSBA) ≥1:5) – Study V72P13E2
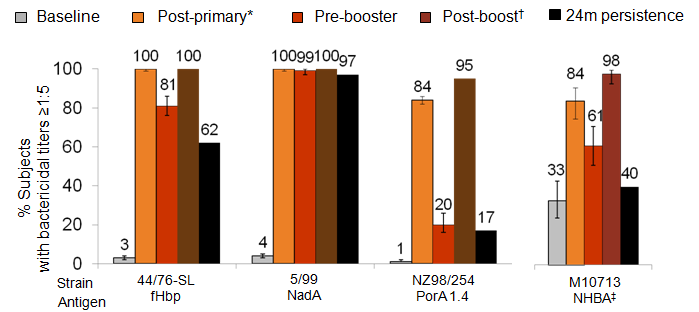
Post-primary: 1 month after 3rd dose, Pre-booster: vaccinees at 12 months of age (6 months after 3rd dose), Post-boost: 1 month after booster, 24m persistence: 12 months after booster dose.
The PBAC noted the rapid waning of titres after primary infant series and after the booster given at 12 months of age. The PBAC noted in a recent publication (Snape MD et al, Persistence of bactericidal antibodies following early infant vaccination with a serogroup B meningococcal vaccine and immunogenicity of a preschool booster dose. 2013 CMAJ 185:E7-24) that there was some anamnestic response at age 40-44 months for infants who received 4 doses. The PBAC considered the key correlate of protection was persistent circulating bactericidal antibodies at adequate levels, which was not supported by the data presented in the submission.
Adolescents, – Primary vaccine schedule:
The submission presented results for the primary endpoint (percentage of participants with hSBA ≥1:4).
The baseline (pre-vaccination) proportion of all trial participants with a titre of ≥1:4 was 30-46%, against fHbp, NadA and PorA P1.4. Analysis of NHBA was a post-hoc analysis, with a small sample size and at baseline a higher percentage of subjects were already seropositive for NHBA. The PBAC considered the effect of the vaccine may be confounded by the high baseline antibody responses.
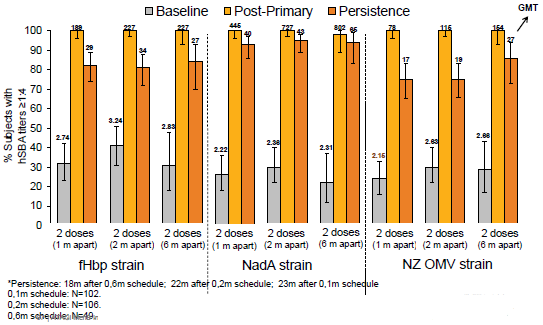
Following two doses, either 1 or 2 months apart (as the proposed NIP schedule), 100% of participants had hSBA titres ≥1:4 one month after the second dose. Ninety-three precent of participants had hSBA titres ≥1:4 one month after a single dose. At 18-23 months after the last 4CMenB vaccine dose when given as a two dose vaccination schedule, 75-95% of participants achieved hSBA titres ≥1:4, for the three antigens examined (fHbp, NadA and PorA P1.4). Persistence of effect varied depending on the antigen, but the variation was less than that observed in infant vaccinees. Persistence of effect was not tested for antigen NHBA.
Persistence 18-23 months post vaccination in adolescents – Study V72P10E1
Herd immunity/reduction of carriage:
Study V72_29 assessed the effect of 4CMenB (2 doses) on the reduction in nasopharyngeal carriage (of all meningococcal B strains (virulent and non-virulent) and all meningococcal ABCWY strains) in university students. Differences at baseline (visit 1), at 1 month after 2nd dose (visit 3), and 3 to 11 months after 2nd dose (visit 4-6) were reported. There was no statistically significant difference in nasopharyngeal carriage prevalence of virulent strains of meningococcal B in the modified intention to treat (MITT) population. The PBAC considered that the prevalence of virulent strains of meningococcal B was most informative for decision making and noted that ATAGI considered it reasonable to apply a lower value than proprosed as the base case efficacy value for reduction of nasopharyngeal carriage in the economic evaluation. The PBAC noted the low absolute value of carriage in the study. The PBAC also noted that the assumption of this analysis is that nasopharyngeal carriage is a surrogate marker for the reduction of transmission of a meningococcal B infection, which can lead to IMD, and the committee considered that it would be difficult to measure herd immunity in terms of reduced IMD in non-vaccinated individuals due to a meningococcal B vaccination program as a) IMD is rare, and b) it would require conducting a large cluster-randomised study in closed environments.
Comparative harms
Infants: In Studies V72P12 and V72P13, the PBAC noted that adverse reactions were not considered severe, and in line with the minor reactions associated with vaccination.
In both studies, fever (≥38.0°C) was reported for 41% to 62% of participants after receiving the 4CMenB vaccine concomitantly with routine vaccines, compared with 17% to 36% after routine vaccinations only. In both studies, the occurrence of medically attended fever ranged from 1% to 3% per visit. Both the TGA and ATAGI noted the concerns associated with high rates of fever seen in the 4CMenB + routine vaccinations group, likely due to pyrogens in 4CMenB.
The PBAC agreed with the Economic Sub-Committee (ESC) and the ATAGI advice that prophylactic paracetamol would be appropriate to reduce this high rate of fever because the evidence presented in the Study V72P16 suggested that prophylactic paracetamol did not substantially affect the immune response of concomitant administration of routine vaccines. The PBAC recalled that there is some evidence that paracetamol given after, rather than before, vaccination can reduce the immune response to routine vaccines (Prymula, R et al. Effect of prophylactic paracetamol administration at time of vaccination on febrile reactions and antibody responses in children: two open-label, randomised controlled trials, Lancet, (2009) 374:1339-1350). However, the ATAGI advice stated ‘It is difficult to predict the likely compliance of prophylactic administration of paracetamol to infants who receive other NIP vaccines concurrently with 4CMenB’ and ‘ATAGI is not confident that a high compliance could be achieved’. The PBAC agreed that uptake and safe compliance of paracetamol was unknown and may be addressed by monitoring of compliance and parent/carer education.
Taking the results of both studies together, 0.7% of participants that received 4CMenB had a febrile seizure, compared to 0.3% of participants that received routine vaccinations only. ATAGI considered that ‘it cannot be concluded whether use of 4CMenB in toddlers may increase the risk of febrile seizures’ (pre-submission ATAGI advice).
Adolescents: The PBAC noted in the trial that less than 5% of the participants across the vaccine groups reported fever (≥38oC) and there was no difference in the percentage of participants reporting fever between the groups that received 4CMenB and placebo.
9. Clinical Claim
The submission described 4CMenB as superior to non-vaccination in terms of comparative effectiveness, with an acceptable tolerability profile in infants and adolescents.
The PBAC agreed that the vaccine is effective in inducing antibodies against the component antigens of 4CMenB. However, in the context of a population based intervention against IMD, the committee considered the clinical claim was highly uncertain, because of the likely short persistence of the antibody response, the unknown effect upon carriage of the bacteria and the overall uncertain long-term protective efficacy against infection and disease.
10. Economic Analysis
The submission presented a modelled economic evaluation (cost utility analysis) based on the claim of superior efficacy and/or safety.
A dynamic transmission model was used to estimate the number of individuals infected with vaccine-preventable meningococci and the number of cases of invasive meningococcal B disease (IMD) that occur. The model consisted of nine mutually-exclusive states, involving a combination of: whether seroprotected; whether infected with vaccine-preventable or non-vaccine-preventable meningococci (or neither); and IMD. All vaccine-preventable strains have been grouped together, and consequently all transmission and infection parameters are assumed to represent an average across all vaccine-preventable strains.
The submission modelled six vaccination scenarios, representing different combinations of routine infant and adolescent vaccination with or without catch-up programmes:
1. routine infant schedule (four doses at 2, 4, 6 and 12 months of age);
2. routine adolescent schedule (two doses at 15 years of age);
3. routine infant and adolescent schedules;
4. routine infant and adolescent schedules with catch-up for older infants and toddlers (two to three doses);
5. routine infant and adolescent schedules with catch-up for adolescents (two doses); and
6. routine infant and adolescent with catch-up for older infants and toddlers and adolescents.
The PBAC agreed that the submission’s proposed base case, scenario 6, was the most clinically appropriate scenario for decision making.
The submission presented an ICER of between $45,000 and $75,000 per quality adjusted life year (QALY) for routine infant and adolescent vaccination with catch-up for older infants and toddlers and adolescents versus no vaccination. This is based on a dynamic transmission model, taking the hSBA outcomes (the trial basis of the efficacy point estimates were not specified), applying a modelling horizon of 100 years, and applying utility weights from several published studies and differential annual discount rates for costs and for health outcomes. The PBAC noted that the ICER/QALY was highly sensitive to the discount rate and reiterated that discounting as outlined in its guidelines with sensitivity analysis is most informative for decision making. The PBAC considered that the economic claim is highly uncertain.
The level of concordance between the strains causing meningococcal disease against which 4CMenB is effective and those in circulation in Australia was measured using the Meningococcal Antigen Typing System (MATS) developed by the sponsor. Isolates (n=373) from IMD cases in all States/Territories (excluding Victoria, and 20 from WA) during the period January 2007 to December 2011 were analysed. Isolates that met a minimum threshold of reactivity (fHbp, NHBA and NadA, quantified by ELISA and relative potency compared to a reference strain) or the presence of PorA P1.4 (genotyping) were deemed to be “covered by 4CMenB”. From these data, an estimated 75.9% (95% CI: 63.3%, 86.9%) of isolates would be covered by bactericidal antibody responses to 4CMenB vaccine. The PBAC noted that MATS predicts killing by pooled sera from vaccines shortly after full vaccination. While there is some data to correlate MATS predicted killing with strain coverage, there is no data to confirm correlation between MATS estimated coverage with actual vaccine efficacy. The PBAC noted that MATS provides no information on the proportion of vaccinees who fail to achieve protective titres. The PBAC noted, notwithstanding the technical issues of measuring the NadA coverage with MATS and that seroresponse against two or more antigens would provide a greater degree of certainty of coverage, that the immune response was most robust against NadA, which had the lowest individual coverage.
|
|
Coverage (%) of individual antigen by MATS (Figure C.2.2 of submission) |
% participants with bactericidal titres ≥1:5 ‘24m persistence’, Figure 1 (Study V72P13E2) |
|---|---|---|
|
fHbp |
48.0% |
62% |
|
NHBA |
57.1% |
40% |
|
NadA |
0.5% |
97% |
|
PorA |
22.3% |
17% |
The MATS estimate of coverage might be a reasonable surrogate for clinical efficacy when all vaccinees achieve a protective response against all antigens, but predicting efficacy based on MATS estimated coverage becomes increasingly uncertain if antibody responses are heterogeneous and as antigen-specific titres wane over time. This factor added to the uncertainty of extrapolating vaccine efficacy from the immunogenicity and MATS coverage data.
The submission presented persistence data from V72P13E2, V72P12E1, V72P9E1, V72P6E1 and V72P10E1. The submission assumed that waning follows an exponential curve: an individual is sero-protected against a strain at time ‘t’ if the predicted antibody titre against any of the four antigens expressed by that strain at time t remains above the bactericidal threshold. The PBAC noted that the method for estimating the mean duration of protection against each antigen remained unclear and that the estimates may be inaccurate because of the limited follow-up time in the trials and the uncertain correlation between immunological surrogate and protection.
The model used a 100-year time horizon. The PBAC noted that, with this time horizon and the annual discount rate for outcomes, the model: 1) favours the vaccine in capturing herd effects; and 2) is sensitive both to changes in serotype prevalence over time and also to improvements in management/ decreased morbidity (from disease and sequelae) over time.
The submission’s base case economic model assumed a value of 50% for vaccine efficacy on carriage acquisition - the midpoint between a subgroup analysis of carriage study V72_29 and Maiden & Stuart (2002). Maiden & Stuart (2002) was an observational study examining meningococcal C conjugate vaccination which found a 66% overall reduction in meningococcal C carriage in young adults. Both ESC and ATAGI considered that it was inappropriate to extrapolate data regarding meningococcal C conjugate vaccines to 4CMenB. Both committees considered the data insufficient to support certainty in prediction of herd effects of immunisation in economic analyses and whether the impact on carriage in young adults is applicable to infants. The PBAC agreed with these committees that the claim of the size of the herd immunity effect was highly uncertain, based on the data provided. The sponsor noted the ESC and ATAGI advice and, in the pre-PBAC response, reduced the value for carriage acquisition in the model, the point value of efficacy, 3-11 months after the 2nd dose in the V72_29 study. The sponsor also offered a price reduction.
The assessment of the vaccine’s effect on quality of life was based on utility values for the long-term sequelae taken from several published studies. The PBAC noted that most sources of the utility values and other model inputs were provided before the PBAC meeting. The PBAC noted that no disutilities were applied for adverse events. The PBAC noted that ESC considered it is appropriate not to apply disutilities for febrile events, assuming that prophylactic use of paracetamol is effective, but considered that this assumes that adherence to this recommendation would be high. As paracetamol does not prevent febrile convulsions, the PBAC considered that these adverse events should be included as febrile seizure can lead to long-term sequelae.
The PBAC noted that the estimates in the model for vaccine efficacy before completion of primary infant 3-dose course were 0.42 and 0.61 after one and two doses, respectively. These values were based on little or no data and the method of calculating the estimates from data on the 4 antigens was not presented.
The PBAC noted that individuals who are sero-protected without vaccination (ie natural immunity) could not enter the sero-protected state. The PBAC considered that it was likely that pre-vaccination sero-protection was not taken into account and that this was not appropriate. The PBAC noted that at least 30-45% of adolescents in the Study V72P10 (conducted in Chile) were already sero-protected at baseline (80-96% against NHBA-containing strains), but they would only enter the sero-protective state following vaccination, which favours the vaccine. The PBAC noted that, in a recent study in the UK, the baseline sero-protection levels in young children (40-44 months of age) were reported as 63% (46-77) for fHpb, 3% (0-13) for NadA, and 0% (0-9) for PorA (Snape, 2013). The PBAC noted in the ATAGI advice that only a small proportion of Australian adolescents (aged 11–17 years) who participated in a study had baseline bactericidal titre of ≥1:5 against the indicator strains (7%, 1% and 0% against, fHbp and NadA, PorA respectively). baseline levels varied by antigen, by age (infants vs adolescents) and by geographic region. The PBAC considered additional data on natural immunity to the antigens of 4CMenB in Australia would be informative to the economic evaluation.
The PBAC noted that changing the assumptions from those originally submitted in the economic model to those in the pre-PBAC response resulted in minor changes to the ICER/QALY. However, when recalculated with annual discount rates of 5.0% costs/5.0% outcomes, the ICER for the base case Scenario 6 was greater than $200,000/QALYG.
The PBAC noted from sensitivity analyses of discounting and price, using the pre-PBAC response model, that a further price reduction would be necessary to give an ICER/QALY in the range that the PBAC has accepted for other vaccines it has recommended for inclusion in the NIP.
11. Estimated PBS Usage and Financial Implications
The submission estimated that over 4 million children and adolescents would be vaccinated over the first 5 years of a full vaccination program.
The PBAC noted the crude estimates provided by ATAGI of the number of invasive meningococcal B disease (IMD) cases, deaths and sequelae that could potentially be prevented through implementation of a 4CMenB immunisation program. These are presented in the table below. The PBAC noted that the estimates were based on the following assumptions: diagnosis of meningococcal B in 2006-2011, indirect herd protection effect was not taken into account, no waning of vaccine effectiveness would occur in the first 5 years, vaccine efficacy and strain coverage were based on the results of a number of clinical studies, vaccine uptake (coverage) assumptions were based on previous experience of similar vaccine schedules in Australia.
|
Age group |
Cumulative total after first 5 years of program |
Cumulative total after first 10 years of program |
||||
|---|---|---|---|---|---|---|
| Number of MenB IMD cases prevented | Number of MenB IMD deaths prevented | Number of patients with sequelae prevented | Number of MenB IMD cases prevented | Number of MenB IMD deaths prevented | Number of patients with sequelae prevented | |
|
<1 year |
92 |
5 |
31 |
184 |
9 |
63 |
|
<5 years (incl <1yr) |
154 |
7 |
53 |
370 |
16 |
128 |
|
15–24 years |
70 |
2 |
39 |
211 |
6 |
119 |
|
Total for all ages |
224 |
9 |
93 |
591 |
22 |
250 |
MenB: meningococcal B; IMD: invasive meningococcal B disease
The cumulative cost of the programme over 5 years was estimated to be greater than $400 million.
The PBAC noted that cost of implementing the programme in school may be underestimated. The sponsor assumed that the implementation of 4CMenB in schools would add approximately 1.0% to the overall cost of the vaccinations received at school, while a likely cost could be around 15.5% of the vaccination costs for adolescents, based on the 2004 report from the Municipal Council of Victoria.
12. Recommendation and Reasons
The PBAC did not recommend the inclusion of the 4CMenB vaccine on the National Immunisation Program Schedule for the prevention of meningococcal B disease in infants and adolescents.
The PBAC considered the burden of meningococcal disease, the public concern about rapidly developing and often fatal infection, and that the development of the proposed vaccine may represent a technical advance in the field of vaccinology. The PBAC concluded that, over the first 5 years of the requested NIP listing as proposed by the sponsor: over 4 million children and adolescents would be vaccinated costing the government over $400 million, estimated to prevent 224 cases of invasive meningococcal disease, 9 deaths due to meningococcal B disease, and 93 patients with sequelae. However, the PBAC considered that there was a limited demonstration of and multiple uncertainties in relation to the clinical effectiveness of the vaccine against the disease when delivered in a vaccination program. In addition, the PBAC concluded that the ICER was unacceptability high and was based on uncertain assumptions about extent and duration of effect and herd immunity.
The PBAC noted that the reverse vaccinology employed in the development of the 4CMenB vaccine overcame the considerable block to the development of a vaccine against meningococcal B strains where, unlike non-strain B meningococcal strains, the polysaccharide capsule from the bacterial cell wall is poorly immunogenic and has a structure similar to polysaccharides in the developing human central nervous system.
The PBAC noted the rapid onset of disease following infection and that clinical improvement in diagnosis and management of the infection has had little impact on IMD outcomes. The PBAC noted the strong consumer support for this submission, highlighting the community’s desire to overcome IMD.
The PBAC noted that there are a number of aspects of the clinical data that leads to an uncertain claim of clinical effectiveness.
Firstly, the PBAC noted that no direct evident was presented regarding vaccine efficacy against infection and disease. Due to the low infection rates of meningococcal B, a randomised efficacy study is not feasible. The PBAC accepted that hSBA titre threshold of ≥1:4 is historically a ‘gold standard’ surrogate marker of vaccine efficacy, and this titre was accepted in the consideration of the combination vaccine for Haemophilus influenzae and Neisseria meningitis serogroup C. The PBAC noted that the threshold titre value was determined based on the polysaccharide capsule, not bacterial proteins, as used in the 4CMenB, and considered that the validity of this surrogate outcome and threshold titre of adequate protection had not been addressed in relation to the structure of the components of the proposed vaccine.
Secondly, the PBAC accepted that the data showed a clear immunogenic effect of the vaccine, but considered that the long-term persistence of a protective immune response had not been adequately supported. In the trials of infant vaccinees, antibody titres waned quickly and at different rates, particularly titres against PorA P1.4 and NHBA.
Thirdly, the PBAC considered that ability of the vaccine to generate a protective herd immune response on a population level had not been demonstrated. The PBAC considered that carriage should be estimated based on the evidence on virulent meningococcal B strains, but not other non-B meningococcal strains. The PBAC considered it was not reasonable to use data from a carriage study of a meningococcal C conjugate vaccine (Maiden & Stuart, 2002) to estimate the reduction of carriage acquisition, given the differences of bacterial strains and vaccine components (polysaccharide vs protein). The PBAC accepted the ATAGI advice of the vaccine’s efficacy against nasopharyngeal carriage, based on the V72_29 study. The PBAC considered that there is insufficient evidence to accept that the carriage study presented in the submission (of 1,958 university students, (150 individuals with virulent meningococcal B)) is applicable to infants. The PBAC noted in a recent publication of another vaccine against meningococcal B (Delbos V et al, Impact of MenBvac, an outer membrane vesicle (OMV) vaccine, on the meningococcal carriage, 2013, Vaccine, 31:4416-20) that, during a meningococcal B outbreak, the carriage rate was 1.2% (9 confirmed meningococcal B in 761 unvaccinated children, aged 1-7).
Fourthly, the PBAC noted that the submission estimated that 75.9% (95% CI: 63.3%, 86.9%) of clinical isolates would be covered by bactericidal antibody responses elicited by 4CMenB, using the MATS. The PBAC was concerned that this typing system was an in vitro method and that the basis of the claim that MATS is a correlate of protection is not clear. The PBAC noted that assay was carried out on pooled immune sera of infants taken 1 month after the 4th dose of 4CMenB, at the likely peak of the antibody response. The PBAC agreed that the MATS estimate of coverage might be a reasonable surrogate for clinical efficacy when all vaccinees achieve a protective response against all antigens, but the committee considered that predicting efficacy based on MATS estimated coverage becomes increasingly uncertain if individual antibody responses are heterogeneous and as antigen-specific titres wane over time. The PBAC, noting that 36.7% (upper bound of the 95% CI) of meningococcal B strains may not be covered by the vaccine, considered that monitoring of long-term variation of meningococcal B strains in the community was an important issue to be addressed in any implementation of a vaccine programme.
The PBAC accepted in general that the local transient reactogenicity of 4CMenB was not different to routine vaccination. The PBAC noted the rates of fever in infant participants of the trials receiving 4CMenB and agreed with the ATAGI that prophylactic paracetamol would be appropriate, as the evidence presented in the submission suggested that prophylactic paracetamol did not substantially affect the immune response of concomitant administration of routine vaccines. The PBAC recalled that prophylactic paracetamol was recommended with the use of the diphtheria-tetanus-whole cell pertussis vaccine (DTPw), before the acellular vaccine was introduced on the NIP. The PBAC noted that ATAGI was unable to identify reliable data or studies on compliance with the recommendation for prophylactic use of paracetamol. The PBAC agreed with ATAGI that it is difficult to predict the likely compliance of prophylactic paracetamol. Given that paracetamol does not prevent febrile convulsions, the PBAC considered that parent/carer education, surveillance of the use of paracetamol and of emergency department admissions for febrile convulsions were important issues to be addressed in any implementation of a vaccine programme. This would help minimise the risk of reduced coverage rates for infant vaccination overall due to loss of confidence in immunisation arising from concerns about increased rates of fever and convulsions.
The PBAC considered that, given its questions about the clinical efficacy of 4CMenB, the economic evaluation was highly uncertain due to the assumptions used in the model and the proposed price of the vaccine.
The PBAC noted that the estimates in the model for vaccine efficacy before completion of primary infant 3-dose course were 0.42 and 0.61 after one and two doses, respectively. The source of these estimates was not presented in the submission, but the PBAC accepted the advice from ATAGI that considered the estimate of 0.61 was reasonable and the estimate of 0.42 was reasonable but imprecise.
The PBAC accepted the time horizon of 100 years used in the model, in line with other vaccines considered by the PBAC. However, the committee considered that it was not reasonable to assume that, over 100 years, the distribution of strains would not change, that each case would result in the same reduction in quality of life and there would be no improvement in the outcomes of meningococcal disease or in the management of disabilities over this time frame.
The PBAC noted that, in the economic model, individuals who had natural immunity could not enter the sero-protected state, only vaccinated individuals. The PBAC considered that this assumption favoured the vaccine, particularly in the adolescent cohort. The PBAC noted that there was limited data available on the levels of natural immunity to meningococcal B strains in Australia to inform this assumption of the economic model.
The PBAC noted that the submission presented the base case of the economic evaluation with a differential annual discount rate for costs and for outcomes. The ICER/QALY was highly sensitive to the discount rates applied to costs and outcomes. The PBAC considered that, consistent with its consideration of other vaccines for the NIP, it was not reasonable to apply differential discount rates in the base case of the economic model. The PBAC noted the discussion by ESC about discounting rates in economic modelling. The sponsor argued that the application of differential discounting to the economic model was appropriate because ‘the burden mainly occurs at an early age implying the loss of many decades of life and early incident of permanent disability that lasts for the rest of life. In addition, a specific benefit of intervention with 4CMenB, as opposed to non-vaccine interventions, is that there is a disproportionate benefit accumulated over time, as the proportion of the population who are protected increases (herd effect).’ The PBAC did not accept this argument as the long-term efficacy of the vaccine in individuals and the reduction of carriage has not been adequately demonstrated in the submission. The PBAC recalled that differential discounting rates were not used in the deliberation by the PBAC for recommending other vaccines for the NIP, such as for rotavirus (where immediate benefits were expected) and for HPV (where the major benefits begin to accrue approximately 20 years after immunisation). The PBAC considered that NIP listing should only occur at a price that produces an acceptable ICER as a basis for PBAC recommendation.
The PBAC considered that it was not informative to consider a hyperendemic scenario in the economic evaluation for the inclusion of 4CMenB in the NIP, noting that the predictability of a hyperendemic scenario is such that it does not inform routine decision making. The PBAC noted that hyperendemic meningococcal B outbreaks had occurred in New Zealand but did not consider that similar outbreaks were necessarily likely to occur in Australia.
The PBAC noted that there is a higher IMD burden in the Aboriginal and Torres Strait Islander (ATSI) population, particularly in children under 5 years of age, where confirmed cases were 3.8 times higher than their non-Indigenous counterparts for 2006-2011. The PBAC did not consider it appropriate to recommend NIP listing of 4CMenB for use in the ATSI population only given the uncertain clinical efficacy and unacceptable cost-effectiveness of the vaccine. The PBAC also noted the concerns of ATAGI that targeted immunization strategies in this population have achieved suboptimal vaccine coverage compared to universal strategies, that benefits would be realized on an individual not group basis, and that there would be considerable implementation and communication issues to consider around perceived differences in the risk-benefit acceptability of the vaccine for Indigenous versus non-Indigenous populations.
The PBAC noted the successful introduction of the meningococcal C conjugate vaccine into Australia. Confirmed cases of IMD caused by meningococcal C have fallen to almost zero within 10 years of introduction of this conjugate vaccine. The PBAC also noted the recent success in decreasing the incidence of meningococcal disease by a vaccine against the meningococcal A strain in Africa.
The PBAC noted that, in the financial estimates section of the submission, the sponsor estimated over 4 million children would be vaccinated over the first 5 years of a full 4CMenB vaccination programme (including catch ups), at a net cost to the government of greater than $400 million. Based on estimates provided by ATAGI, this vaccination programme would prevent 224 cases of invasive meningococcal disease, 9 deaths due to meningococcal B disease, and 93 patients with sequelae after 5 years. The PBAC concluded that the rarity of invasive meningococcal B disease compared to the large number of vaccinations that are required was the primary driver of the unfavourable incremental cost-effectiveness ratio. The PBAC considered that, given the clinical uncertainties of a population wide prevention, there was high financial risk to the government and reduced opportunity to fund other interventions which are acceptably cost-effective.
The PBAC noted the sponsor’s proposal to support additional research to address and potentially manage uncertainties associated with implementing the requested NIP listing:
- continuation of the MATS analysis;
- an effectiveness study, similar to the observational study protocol (Study V72_38OB) developed with Public Health England, which uses a screening method. Uptake and routine surveillance data for meningococcal disease would allow assessment of the vaccine effectiveness, as well as help provide longer-term answers on geographical strain coverage (by incorporating data from MATS), waning, herd immunity and capsule replacement;
- a vaccine registry;
- a carriage study, such as a school-based study comparing carriage at baseline and after introduction of the vaccine in a national programme. The study’s scope, protocol, choice of investigators and governance could be defined and agreed upon in further discussions with ATAGI.
The PBAC agreed that this research could provide valuable information to address many of the clinical issues raised during the committee’s consideration of the submission.
The PBAC noted that a registry would need to cover an age range of 2 months to early adulthood. In Australia there are two national vaccine registries: the Australian Childhood Immunisation Register (ACIR, up to the age of 7) and the National HPV Vaccination Program Register (adolescents to early adulthood). The PBAC considered the expansion of the ACIR to monitor immunisation coverage levels for the whole of life would be preferable option over the creation of a third registry.
The PBAC noted that a controlled intervention study in the Oxford Health region of a Hib conjugate vaccine was completed in 1991 to address concerns of adding Hib conjugate vaccines to the UK national immunisation programme. Routine immunisation with Hib conjugate was introduced in October 1992 in the UK, resulting in near elimination of Hib disease (review by Heath & McVernon (Heath, PT & McVernon, J.The UK Hib vaccine experience. Arch Dis Child, 2002: 86:396-99)). The PBAC advised that consideration should be given to conducting a cluster randomised controlled trial (possibly clustering at State and Territory level) as a robust method of addressing uncertainties of the clinical effectiveness of the vaccine. If feasible, the PBAC considered that such a trial should be included as a component of the sponsor-supported additional research.
The PBAC considered whether a managed entry approach would be appropriate for the 4CMenB vaccine to accommodate these research proposals. Co-implementation with the sponsor of such a scheme would likely address the extent and persistence of vaccine effectiveness, the extent of the reduction of nasopharyngeal carriage leading to extent of herd immunity, the surveillance for reactogencity and the requirement of an additional booster dose at age of 4 or in early adulthood.
However, the PBAC held grave concerns that if NIP listing was implemented in the context of a managed entry scheme, and the research subsequently showed that the expected benefits were not realised, there would be great difficulty associated with disinvestment and removal of the vaccine from the NIP, and such an event may undermine public confidence in immunisation in general.
Recommendation:
Rejected
13. Context for Decision
The PBAC helps decide whether and, if so, how medicines should be subsidised in Australia. It considers submissions in this context. A PBAC decision not to recommend listing or not to recommend changing a listing does not represent a final PBAC view about the merits of the medicine. A company can resubmit to the PBAC or seek independent review of the PBAC decision.
14. Sponsor’s Comment
Novartis is disappointed by the decision of the PBAC not to recommend Bexsero® for inclusion onto the National Immunisation Programme (NIP) at this time. Meningococcal B is the single leading cause of bacterial meningitis and sepsis in Australian infants and teenagers, accounting for approximately 85% of all meningococcal disease cases. The disease is easily misdiagnosed in its early stages and can develop rapidly, frequently leading to death or permanent disability within 24 hours of onset of symptoms.
Novartis is committed to the inclusion of Bexsero® on the NIP, since we believe that widespread use of the vaccine will significantly reduce the risk of this devastating disease in the Australian community. Novartis has carefully considered the PBAC’s commentary on the submission and is working with the Committee to address all outstanding areas of uncertainty in a resubmission to the PBAC submitted March 2014.




