EAPD Quick Reference Guide
Please note: The information on this page is no longer current and is only included here for historical reference.
Current information is maintained on the Price Disclosure (SPD) page.
PDF printable version of this page (PDF 149KB)
Purpose
This document provides an overview of requirements under the Pharmaceutical Benefi ts Scheme (PBS) Expanded and Accelerated Price Disclosure (EAPD).
This document can be read in conjunction with the Expanded and Accelerated Price Disclosure Procedural and Operational Guidelines (PDF 149KB).
What is Price Disclosure?
Price disclosure is a key component of the PBS reforms package, having commenced in August 2007. The price disclosure program progressively reduces the price of some PBS medicines which are subject to competition, ensuring better value for money from these medicines.
Under price disclosure, companies submit sales information to the department, and, based on this information, the price the Australian Government pays is adjusted to refl ect more closely the price at which the medicines are supplied.
What is Expanded and Accelerated Price Disclosure (EAPD)?
EAPD commences on 1 December 2010 and is an extension of the price disclosure program. EAPD will include mandatory disclosure for all sales of non exempt medicines on F2 (see www.pbs.gov.au).
Medicines under price disclosure arrangements prior to 1 December 2010 will continue to disclose under transitional arrangements.
Expanded and Accelerated Price Disclosure process1 ‘First Main Disclosure Cycle’
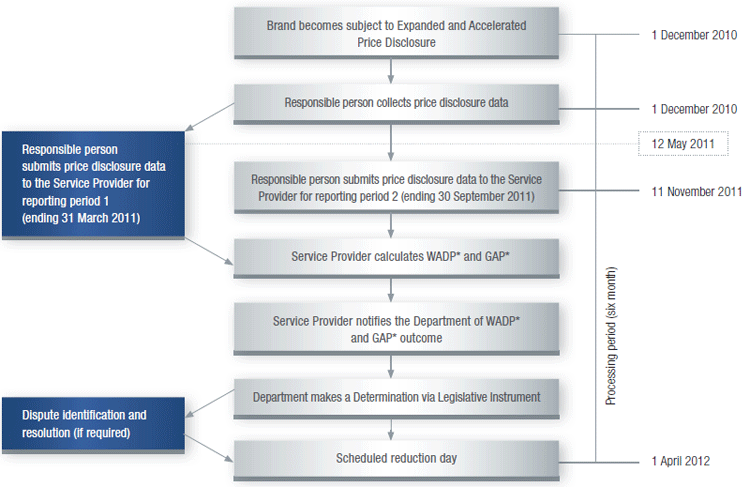
* GAP – Guaranteed Adjustment Proportion calculation applies to fi rst main cycle
only
* WADP – Weighted Average Disclosed Price calculation
Data Collection
What data must be collected?
Responsible persons must collect and submit the following brand specifi c data in line with EAPD requirements:
- the sales revenue for the brand;
- the volume of the brand sold, based on the number of packs sold;
- the kind of incentives (if any) given for the brand for the reporting period; and
- the value of the incentives given for the brand for the reporting period.
NOTE:
- Sales for Exempt items should be excluded.
- Data relating to sales of PBS items to public hospitals must be excluded from the data submitted. All other sales of PBS items must be included. This includes sales of PBS items to private hospitals and over the counter PBS items (whether or not supplied under the PBS)
A list of exempt items is available on the PBS website.
Data collection cycles
The EAPD cycles consist of:
- first Main Disclosure Cycle, (this is the only cycle that the Guaranteed Adjustment Proportion calculation will be applied to);
- the Interim Supplementary Disclosure Cycle;
- Subsequent Main Disclosure Cycles;
- Supplementary Disclosure Cycles A; and
- Supplementary Disclosure Cycles B.
With the exception of the first Main Disclosure Cycle each EAPD cycle will be no less than eighteen months and will consist of:
- a data collection period of at least 12 months; and
- a six month processing period.
For more information see Fact Sheet 1: Expanded and Accelerated Price Disclosure Cycles.
Transitional Cycles
Three of the data collection cycles under the price disclosure program, prior to 1 December 2010, will be in progress (still in the data collection phase of the cycle). These cycles will become known as the Transitional Cycles.
For more information see Fact Sheet 2: Transitional Cycles.
Data Submission
When to submit data
Data submissions collected for each EAPD cycle must be submitted within six weeks after the end of each of the reporting periods.
How to submit data
The Responsible Person must submit data to the Service Provider. Data submissions will be submitted electronically via direct input or transfer of a data fi le (Excel or XML) to the Service Provider.
NOTE: Data submissions must be accompanied by the EAPD Declaration signed by the Responsible Person’s Authorised Representative as nominated to the Department.
Managing incorrect data or errors
The Service Provider will process all submissions and send a Submission Confi rmation Report to the Responsible Person’s Authorised Representative.
The Authorised Representative should check the confi rmation report thoroughly to ensure that the information is accurate and complete.
If an error is identifi ed the Responsible Person must resubmit the data for the brand of drug as soon as possible. Once submitted, the Service Provider will repeat the confi rmation process.
The accuracy of data submissions provided to the service provider is extremely important, data submissions form the basis for the Weighted Average Disclosed Price (WADP) calculation and for the fi rst main disclosure cycle the Guaranteed Adjustment Proportion calculation. Authorised Representatives are urged to take the necessary steps to ensure data supplied to the service provider is true and correct.
Calculation
The Service Provider will use the submitted data relating to a specifi c drug to calculate a weighted average disclosed price (WADP) for all forms and strengths of that drug that have the same manner of administration. The Department will use this information to determine whether a price reduction will occur.
Possible Outcomes
If the weighted average percentage difference is:
- ? 10%, the approved ex-manufacturer price of each PBS item will be reduced by the weighted average percentage difference to give a weighted average disclosed price.
- < 10%, there is no price change and the approved ex-manufacturer price remains the same.
NOTE:
- The scheduled reduction days for EAPD are:
- 1 April;
- 1 August; and
- 1 December. - A price reduction will occur on the fifi rst scheduled reduction day after the Department has legally determined (via legislative instrument) the price reductions.
Guaranteed Adjustment Proportion (GAP) Calculation
For the first main disclosure cycle, if the guaranteed average saving of a 23% price reduction across all non exempt F2 drugs that become subject to price disclosure on 1 December 2010 is not achieved via the application of the WADP calculation, then the Guaranteed Adjustment Proportion (GAP) calculation will be applied to all drugs whose weighted average percentage was equal to or greater than 10%.
The GAP will proportionally reduce the price of drugs (with a weighted average percentage equal to or greater than 10%) until an average saving of 23% is achieved across non exempt F2 drugs that become subject to price disclosure on 1 December 2010.
The GAP calculation may need to be performed more than once to achieve the guaranteed average savings of 23%.
If a drug reaches its lowest disclosed price (the disclosed price is the price at which the Responsible Person sells the drug, it is calculated from the sales, volume and incentive data provided by the Responsible Person) it will be reduced to that price, and removed from any further iterations of the GAP calculation.
NOTE:
- The guaranteed 23% price reduction will only apply to the fi rst main price disclosure cycle.
- Drugs whose weighted average percentage is calculated to be less than 10% are not included in the GAP calculation.
- No drug will have its price reduced below the lowest disclosed price.
Compliance
Responsible Persons should be aware that there are penalties for noncompliance with the EAPD requirements. For further information regarding a Compliance Culture see the Expanded and Accelerated Price Disclosure Procedural and Operational Guidelines (PDF 149KB).
Compliance requirements are consistent with Price Disclosure policy prior to 1 December 2010.
Further Reference Material
For more information refer to:
- Expanded and Accelerated Price Disclosure Procedural and Operational Guidelines (PDF 149KB)
- Fact Sheet 1: Expanded and Accelerated Price Disclosure Cycles
- Fact Sheet 2: Expanded and Accelerated Price Disclosure Transitional Cycles
- Pharmaceutical Benefi ts Scheme (PBS) website: www.pbs.gov.au
- National Health Act 1953: www.comlaw.gov.au
FURTHER QUESTIONS?
If you have any further questions regarding the Expanded and Accelerated Price Disclosure arrangements please email the Department of Health at eapd@health.gov.au or call the EAPD Inquiry Line on (02) 6289 2303.
1 Based on the first main disclosure cycle commencing 1 December 2010
2 The first main disclosure cycle commencing 1 December 2010 will be 16 months, based
on a 10 month data collection period (made up of one 4 month and one six month reporting
period) and a six month processing period.
3 The Department will be able to provide more information about the Service Provider
once the tender process has been completed.
4 This may involve downloading software provided by the Service Provider. More information
will be provided relating to this process as soon as possible.
5 Expanded and Accelerated Price Disclosure Procedural and Operational Guidelines will
be available on www.pbs.gov.au by 26 November 2010.
6 As above




