Australian Statistics on Medicines 2010
Acknowledgments
Prepared by Vanna Mabbott, Maxine Robinson, Alicia Segrave and Quinton Brennan of the Drug Utilisation Sub-Committee Secretariat.
We would like to thank the following people for their help in the access and provision of data and information used in this report:
- The World Health Organization Collaborating Centre for Drug Statistics Methodology.
- Joanna Wilson, Governance and Access, Department of Health.
- Katherine Gray, Advisory Committee on the Safety of Medicines, Therapeutic Goods Administration.
- Kate Ridgway, Australian Institute of Health and Welfare.
Australian Statistics on Medicines 2010 edition
ISBN: 978-1-74241-827-8
Online ISBN: 978-1-74241-828-5
Publications approval number: D0909
Copyright Statements:
Paper-based publications
© Commonwealth of Australia 2012
This work is copyright. You may reproduce the whole or part of this work in unaltered form for your own personal use or, if you are part of an organisation, for internal use within your organisation, but only if you or your organisation do not use the reproduction for any commercial purpose and retain this copyright notice and all disclaimer notices as part of that reproduction. Apart from rights to use as permitted by the Copyright Act 1968 or allowed by this copyright notice, all other rights are reserved and you are not allowed to reproduce the whole or any part of this work in any way (electronic or otherwise) without first being given the specific written permission from the Commonwealth to do so. Requests and inquiries concerning reproduction and rights are to be sent to the Online, Services and External Relations Branch, Department of Health, GPO Box 9848, Canberra ACT 2601, or via e-mail to copyright@health.gov.au.
Internet sites
© Commonwealth of Australia 2012
This work is copyright. You may download, display, print and reproduce the whole or part of this work in unaltered form for your own personal use or, if you are part of an organisation, for internal use within your organisation, but only if you or your organisation do not use the reproduction for any commercial purpose and retain this copyright notice and all disclaimer notices as part of that reproduction. Apart from rights to use as permitted by the Copyright Act 1968 or allowed by this copyright notice, all other rights are reserved and you are not allowed to reproduce the whole or any part of this work in any way (electronic or otherwise) without first being given the specific written permission from the Commonwealth to do so. Requests and inquiries concerning reproduction and rights are to be sent to the Online, Services and External Relations Branch, Department of Health, GPO Box 9848, Canberra ACT 2601, or via e-mail to copyright@health.gov.au.
FOREWORD
It is a great pleasure to introduce the 16th Edition of the Australian Statistics on Medicines. This publication provides an extensive and extremely valuable set of statistics on the use of prescription medicines in Australia up to and including the year 2010. Data in this edition were obtained from the Pharmaceutical Benefits Scheme, the Repatriation Pharmaceutical Benefits Scheme and an ongoing survey of a representative sample of community pharmacies.
Continuous data on the use of prescription medicines are available from 1990. Knowledge about the usage, cost and trends over time in prescription medications is clearly relevant for all four aspects of the National Medicines Policy: access to medicines; quality, safety and efficacy of medicines, quality use of medicines; and a responsible and viable medicines industry. The data are routinely scrutinized by the Drug Utilisation Sub-Committee (DUSC). DUSC is one of the longest-standing committees in the health system, having been convened in 1988 to advise the Pharmaceutical Benefits Advisory Committee and other stakeholders within the National Medicines Policy Framework about the use (predicted and actual) of medicines in Australia. This advice is becoming even more crucial as the number, usage, cost and complexity of prescription medicines increase dramatically with the ageing population and seemingly never-ending development of new medicines. Data from the Australian Statistics on Medicines and advice from DUSC provide an integral contribution to the monitoring and quality assurance of the Pharmaceutical Benefits Scheme, prescribers and the medicines industry.
This edition of the Australian Statistics on Medicines was prepared by staff of the Drug Utilisation Sub-Committee Secretariat. I acknowledge their skill and expertise and thank them for their extraordinary commitment and hard work. The Australian Statistics on Medicines has a critical role in our health care system and provides information that ultimately improves the health of Australians.
David Le Couteur
FRACP PhD
Chair, Drug Utilisation Sub-Committee
- FOREWORD
- INTRODUCTION
- INFORMATION ON THE AUSTRALIAN STATISTICS ON MEDICINES
- Overview
- Pharmaceutical Benefits Advisory Committee
- Drug Utilisation Sub-Committee
- Medicare Australia processing
- Pharmacy Guild Survey data
- Combined database
- ADVERSE DRUG REACTIONS REPORTING IN AUSTRALIA
- THE HIGHLY SPECIALISED DRUGS PROGRAM
- Overview
- Highly Specialised Drugs Working Party
- Criteria for selection of Highly Specialised Drugs
- Supply of pharmaceutical benefits to remote area Aboriginal Health (AHSs) under Section 100 of the National Health Act
- HEALTH EXPENDITURE TRENDS
- DRUG UTILISATION TRENDS
- TABLES IN THE AUSTRALIAN STATISTICS ON MEDICINES
- CAVEATS
- GLOSSARY OF TERMS
- ATC & DDD Additions and Alterations
- TABLE 1: 2010 COMMUNITY PRESCRIPTION NUMBERS, TOGETHER WITH GOVERNMENT AND PATIENT COSTS FOR PBS LISTED DRUGS
- TABLE 2: COMMUNITY PRESCRIPTION DRUG USE, IN DDD/1000/DAY, FOR 2008 TO 2010
- ATC INDEX 2011
- TABLES AND FIGURES
- List of Tables
- Table A: Highly Specialised Drugs—National Usage and Patient Report in Public Hospitals for the period January 2010 to December 2010
- Table B (i): Total expenditure on pharmaceuticals and other medical non-durables as % total expenditure on health, TEH
- Table B (ii): Total expenditure on pharmaceuticals & other medical non-durables per capita, US$ purchasing power parity
- Table B (iii): Total expenditure on health as % gross domestic product
- Tables C: Prescription numbers by ATC groups
- Table C (i): Subsidised prescriptions (PBS/RPBS)
- Table C (ii): Estimated non-subsidised prescriptions (Survey)
- Table D: Top 10 drugs by defined daily dose/thousand population/day, 2010 (including the contribution of constituents of combination products)
- Table E: Top 10 drugs by prescription counts, 2010
- Table F: Top 10 PBS/RPBS drugs by total cost to Australia, 2010
- List of Figures
INTRODUCTION
The data contained in the 2010 Australian Statistics on Medicines are drawn from two sources. The first is the Medicare Australia records of prescriptions submitted for payment of a subsidy under the Pharmaceutical Benefits Scheme (PBS) and Repatriation Pharmaceutical Benefits Scheme (RPBS). The second is an ongoing survey of a representative sample of community pharmacies, which provides an estimate of the non-subsidised use of prescription medicines in the Australian community. The usage of prescription medicines dispensed to in-patients in public hospitals is not available in this report. The usage of subsidised PBS/RPBS prescription medicines to out-patients and discharged patients in four states of Australia and one territory are included. It is planned that all out-patients and discharged patients will receive PBS subsidised prescriptions in the future. The units of measurement are the prescription and the defined daily dose per 1000 population per day (DDD/1000 population/day). The defined daily dose is established by the World Health Organization Collaborating Centre (WHOCC) for Drug Statistics Methodology on the basis of the assumed average dose per day of the drug, used for its main indication by adults. The drugs presented in this publication are arranged using the Anatomical Therapeutic Chemical (ATC) classification system. For more detail on this classification and the unit of measurement, please read the chapter ‘Information on the Australian Statistics on Medicines’. The data are presented in two major tables. Table 1 includes 2010 community (i.e. subsidised and non-subsidised) prescription numbers. These figures are presented together with the government and patient costs for drugs PBS listed and subsidised by the Australian Government only. Cost information on the dispensing of drugs not listed on the PBS and drugs that are PBS-listed but for which no subsidy is claimed from the Australian Government is not available for the period of this report. Collection of under general co-payment PBS volume data commenced on 1 April 2012, and will inform future reports thereafter. Table 2 includes community prescription drug use, in DDDs/1000 population/day, for the years 2008, 2009 and 2010. Table 2 reports the DDDs for each drug, reporting the use in monocomponent (‘plain’) and in fixed dose combination formulations.
INFORMATION ON THE AUSTRALIAN STATISTICS ON MEDICINES
Overview
The development, monitoring and promotion of rational and cost-effective use of medications in society are dependent on accurate information on patterns of drug prescription and use. Where use is considered to be inappropriate, drug utilisation data can monitor the impact of educational or regulatory interventions, and can guide the interpretation of pharmacoeconomic analysis1.
In Australia, community prescriptions (i.e. non-public hospital) are dispensed either as private prescriptions, funded by the patient or private health insurer, or under one of two Government subsidisation schemes—the Pharmaceutical Benefits Scheme (PBS) and the Repatriation Pharmaceutical Benefits Scheme (RPBS). These schemes were established to provide the general community (PBS) and returned servicemen and women (RPBS) with access to necessary medicinal products which are affordable, available and of acceptable standards. Since 2002 prescriptions for an increasing number of public hospital outpatients and many medicinal products supplied at discharge for in-patients have been included in the dataset. In 2010, the RPBS was 6.4% of the size of the PBS and a large majority, approximately 94%, of RPBS prescriptions involved PBS listed drugs.
In Australia, a new medicinal drug must gain approval for supply in accordance with the requirements of the Therapeutic Goods Act 1989. Approval is also required to extend the indications of an established drug. Applications are dealt with by the Therapeutic Goods Administration (TGA) and, for prescription drugs, advice is sought from an expert committee. From 1963 to 2009 this advice was provided by the Australian Drug Evaluation Committee (ADEC). ADEC was replaced in January 2010 with the Advisory Committee on Prescription Medicines (ACPM).
Once a prescription drug is approved for marketing, the company concerned usually applies to have the drug listed on the PBS. This is the national scheme available to the Australian community for subsidising the cost of pharmaceuticals. The subsidised cost, particularly for newer drugs, reduces consumers’ out of pocket expenses therefore many companies seek to have the drug listed on the scheme to facilitate viable marketing.
The Pharmaceutical Benefits Advisory Committee (PBAC) makes recommendations to the Australian Government about which drugs should be listed on the PBS. Pre-market evaluation addresses the issues of quality, safety and efficacy, whereas the PBAC considers effectiveness and cost-effectiveness of the product relative to alternatives, as well as the overall cost to the Government. Once the PBAC has recommended a drug for listing on the PBS, the Pharmaceutical Benefits Pricing Authority (PBPA) negotiates the price with the sponsor company. The PBPA consists of government, industry and consumer representatives. After agreement is reached, the Australian Government considers the advice of both the PBAC and the PBPA and makes a decision on whether the drug will be listed on the PBS.
Under the PBS, patient contributions towards medication costs at pharmacies are capped. In 2010, general patients paid the cost of a prescription up to a maximum of $33.30. Pensioner and concession patients paid $5.40 per prescription.
In addition, there is a Safety Net Scheme to protect people with high medication needs. In 2010, once general patients and/or their immediate family incurred $1,281.30 of PBS expenditure (indexed), PBS/RPBS prescriptions for the remainder of the calendar year cost only $5.40 per prescription. Once pensioners and other concession card holders reached the concession safety net threshold of $324 expenditure (indexed), they received all remaining prescriptions in 2010 free of charge.
It is important to note that patients may be required to pay a surcharge if a doctor prescribes a more expensive brand of an item, when there are cheaper, equivalent brands of that item listed on the PBS.
As the general patient co-payment rises, the dispensed price of many cheaper medical products fall under this level. In such cases the patient pays the full price and no claim for payment was transmitted under the PBS for the period of this report. In 2010, under co-payment general prescriptions represented around 17.8% of all community prescriptions. There are also many drugs that are not listed on the PBS or RPBS and are available only on private prescription, with the patient paying the full cost. Private prescriptions represented 7.1% of community prescriptions in 2010.
Pharmaceutical Benefits Advisory Committee
The Pharmaceutical Benefits Advisory Committee (PBAC) is an independent statutory body established on 12 May 1954, under section 100A of the National Health Act 1953. The role of PBAC is to make recommendations and give advice to the Minister about which drugs and medicinal preparations should be made available as pharmaceutical benefits. No new drug may be made available as a pharmaceutical benefit unless recommended by the PBAC.
The PBAC is required by the Act to consider the effectiveness and cost of a proposed benefit compared to alternative therapies. In making its recommendations, the PBAC, on the basis of expected community usage, recommends maximum quantities and repeats, and may also recommend restrictions as to the indications where PBS subsidy is available. When recommending listings, the PBAC provides advice to the PBPA regarding comparison with alternatives or their cost effectiveness.
Drug Utilisation Sub-Committee
In 1988, the PBAC convened the Drug Utilisation Sub-Committee (DUSC) to assist it in making recommendations for listings on the PBS. Its terms of reference are:
- To develop and advise on the mechanisms for the collection, analysis and interpretation of comprehensive data on utilisation of medicines in Australia.
- To advise PBAC on changes in patterns of utilisation of medicines as a consequence of changes in their availability or subsidy restrictions and to review the utilisation of medicines, including but not restricted to expenditure impacts within the Pharmaceutical Benefits Scheme (PBS).
- To advise stakeholders within the National Medicines Policy framework on the interpretation of patterns of utilisation of medicines, including by placing the results of the data in the context of the limitations of the data.
- To identify potential problems and benefits related to patterns of utilisation of medicines.
- To evaluate policy and other interventions related to the use of medicines.
- To facilitate and promote the dissemination of information on utilisation of medicines.
- To conduct international comparisons of utilisation of medicines by interaction with appropriate international bodies.
National Medicines Policy
The National Medicines Policy (NMP) is a broad framework that aims to improve health outcomes for all Australian’s through access to and appropriate use of medicines.
The overall aim of the NMP is to meet medication and related service needs, so that both optimal health outcomes and economic objectives are achieved. In this context “medicines” means prescription, non-prescription and complementary healthcare products.
The NMP has four central objectives:
- timely access to the medicines that Australian’s need, at a cost individuals and the community can afford;
- medicines meeting appropriate standards of quality, safety and efficacy;
- quality use of medicines;
- and maintaining a responsible and viable medicines industry.
Further information on the National Medicines Policy is available at:
http://www.health.gov.au/internet/main/publishing.nsf/Content/National+Medicines+Policy-1
Drug Classification
The DUSC and the Department of Health have adopted the Anatomical Therapeutic Chemical (ATC) code as recommended by the World Health Organization (WHO). It has been a goal of WHO to have an internationally accepted classification for presenting and comparing drug usage data. In 1982, the WHO Collaborating Centre for Drug Statistics Methodology (WHOCC), located in Norway, was established as a central body responsible for co-ordinating ATC use.
The ATC code itself is a seven digit alpha-numeric code, structured in five levels, that classifies drugs according to their site of action and therapeutic and chemical characteristics.
The first level of the code is the anatomical main group. There are 14 anatomical main groups. The second and third levels are for the therapeutic subgroup and pharmacological subgroup, respectively, with a fourth level being either a chemical or therapeutic subgroup. The fifth level is the actual chemical substance.
The five levels thus are:
| 1 | anatomical main group |
| 2 | pharmacological/therapeutic subgroup |
| 3 | chemical/pharmacological or therapeutic subgroup |
| 4 | chemical/pharmacological or therapeutic subgroup |
| 5 | chemical substance (generic drug name) |
For example, risperidone has the following code: N 05 A X 08.
| N denotes | Nervous system |
| 05 | Psycholeptics |
| A | Antipsychotics |
| X | Other antipsychotics |
| 08 | Risperidone |
ATC system main groups:
The 14 anatomical main groups of the ATC code are:
| A | Alimentary tract and metabolism |
| B | Blood and blood forming organs |
| C | Cardiovascular system |
| D | Dermatologicals |
| G | Genitourinary system and sex hormones |
| H | Systemic hormonal preparations, excluding sex hormones and insulins |
| J | Anti-infectives for systemic use |
| L | Antineoplastic and immunomodulating agents |
| M | Musculo-skeletal system |
| N | Nervous system |
| P | Antiparasitic products, insecticides and repellents |
| R | Respiratory system |
| S | Sensory organs |
| V | Various |
Although the ATC code extends to the generic drug level, it does not identify dosage forms, pack sizes, strengths or brand names.
The WHOCC, together with the Nordic Council on Medicines, undertakes regular revisions of the ATC system. They receive expert advice from an advisory board and an established procedure exists to manage requests for new classifications and to regularly review the current structure. Changes implemented in 2011 are included in the Anatomical Therapeutic Chemical Index (ATC) & Defined Daily Dose (DDD) additions and alterations section in this publication.
Measurement Unit
The international unit of drug utilisation adopted by the DUSC to accompany this coding system is the defined daily doses, per thousand of the population, per day (DDDs/1000/day). The defined daily dose is established by the Nordic Council on Medicines and the WHO Drug Utilisation Research Group on the basis of the assumed average dose per day of the drug, when used for its main indication by adults3.
Use of DDDs allows for comparisons of drug utilisation independent of differences in price, preparation and quantity per prescription. It also allows comparison of the use of drugs in different therapeutic groups, and between regions and countries. Expressing drug use in DDDs/1000/day allows the aggregation of data for those drugs which have differing daily doses. The DDD, however, is only a technical unit of use and does not necessarily reflect the recommended or average prescribed dose in Australia.
The DDDs/1000/day figure is calculated from prescription data in the following way:
| N x M x Q x 1000 |
| DDD x P x D |
Where:
| N | is the number of prescriptions dispensed in the year |
| M | is the drug mass in each unit (tablet, capsule, injection, pack etc.) (e.g. milligrams or grams, expressed in the same unit as DDD) |
| Q | is the average dispensed quantity (ie. number of units) per prescription |
| P | is the mid-year Australian population for the year of data collection (see Australian Bureau of Statistics website for population figures used in this edition: http://www.ausstats.abs.gov.au/ausstats/subscriber.nsf/0/ 32F03D06DEBCFA06CA2579190013E303/$File/31010_mar%202011.pdf) |
| D | is the number of days in the year. |
The DDDs/1000/day can be calculated over other time periods such as monthly or quarterly.
For PBS items, the mass amount (M) is the amount of active drug contained in an individual dose unit e.g. tablet, capsule, suppository etc. Non-PBS items are estimated from the Pharmacy Guild survey. The data from the survey does not include information on the quantity supplied per prescription, therefore the mass amount for non-subsidised items is the total amount of active drug contained in the pack.
For prescriptions forwarded for subsidy, the average quantity dispensed (Q), is available from Medicare Australia data. For prescriptions that are priced under the general co-payment, quantity is assumed to be the average quantity of the subsidised prescriptions for that drug (i.e. as concession, safety net and Veterans Affairs (Repatriation) prescriptions). For private prescriptions, the quantity dispensed is assumed to be the retail pack size.
For a chronically administered drug, the DDDs/1000/day figure indicates how many people, per 1000 of the population, may, in theory, have received a standard dose (as defined by the DDD) daily.
For drugs used intermittently, for example anti-infectives, usage expressed in DDDs/1000 /day may similarly give a rough estimate of the average proportion of the population using these drugs every day. To estimate the number of patients treated during the year supplementary information, such as the average duration of treatment, is necessary3.
The ATC/DDD methodology has a number of limitations. All drugs dispensed are not necessarily consumed and the DDDs/1000/day is calculated for the total population, while drug use may be concentrated in certain age groups or a particular sex.
It is difficult to assign a DDD, and on occasions an ATC code, to some preparations that have multiple active ingredients. For some drug groups, such as the dermatological and antineoplastic drugs, highly individualised use and wide dose ranges, as well as the experimental nature of some of the therapy, make it difficult to define a daily dose. Consequently, there may be a delay between the marketing of a drug and the availability of an ATC code and its associated DDD.
Generally agreed indications for use of a drug may be re-evaluated in light of experience with adverse reactions and other pharmacological effects. Drugs may have multiple indications and it may be difficult to determine a preparation’s use. Also, the DDD is based on overseas experience and may not reflect the prescribed adult dose in Australia.
As more medicinal products are listed on the PBS in formulations of two or more combinations the DUSC has considered that it is important to record the contribution, in terms of DDDs, of each constituent where appropriate. Therefore additional information on the contribution of the constituents of combination pharmaceutical items in addition to single component items will be reported in table 2.
Medicare Australia processing
In 1990, the processing of prescriptions submitted for payment of a subsidy under the PBS/RPBS was taken over by the Health Insurance Commission, now the Department of Human Services. Daily data transmissions, containing prescription records that do not allow the identification of an individual patient, are provided by Medicare Australia to the Department of Health for summarisation.
Nevertheless, significant gaps in the data result from the inability to estimate both the level of use for PBS drugs priced under the patient co-payment, and the level of private prescription drug use1.
Pharmacy Guild Survey data
Since 1989, DUSC has commissioned the Pharmacy Guild of Australia to conduct an annual survey to estimate the prescription volumes for drugs in the non-subsidised categories i.e. private prescriptions and PBS prescriptions priced under the general patient co-payment. Total dispensing information is collected each month from pharmacies that are members of the Pharmacy Guild. The sample increased in 2007 from 150 to 370.
Amfac, a major pharmacy computer software supplier, was commissioned to administer the collection of the data in 1988. Under the joint direction of DUSC and the Guild, Amfac contracted a firm of statisticians, specialising in survey design and analysis, to design a stratified random sample, using the Guild membership, which represents approximately 80% of pharmacies in Australia, as the population base. In 1993, the survey sample was reviewed and augmented with the assistance of the Statistical Services Section within the Department. A review of the representativeness, sample size, design and risks for the survey was carried out by the Australian Bureau of Statistics (ABS) in February 2002. It found a small relative standard error, for the sample size of 150 pharmacies, of 4.3%.
In the original form of the survey, dispensing records from the participating pharmacies were sent to Amfac’s Canberra premises. Several hundred diskettes were summarised by drug code and category. A single disk was then forwarded to the Department. Details of the dispensing of individual participating pharmacies are not available on these data.
The Survey data was not supplied by the Guild from September 1999 to February 2001. When monthly data collection recommenced in February 2001, retrospective data was retrieved using an internet connection between each participating pharmacy and a software provider. A total of 142 pharmacies participated in this data collection. An agreement between the software provider, the Guild and Department has been developed to ensure continuation of the Survey.
Following reinstatement of the Survey, data is now transmitted electronically from the participating pharmacy dispensaries to the software providers. Data is forwarded to the Guild and then to DUSC. The data continues to be de-identified with respect to individual pharmacies and individual patient prescriptions.
The pharmacies in the survey are selected to be representative of the population of operational pharmacies with regard to PBS dispensing volume and geographical location, and are similarly stratified. In order to compare and then extrapolate the survey quantities to estimates of use in Australia, the subsidised PBS prescription data supplied by all pharmacies in Australia are stratified into the same four equal dispensing volume ranges based on their annual average PBS dispensing from the previous year. A weighting factor is calculated for each PBS and Amfac item code by comparing the number of pharmacies and PBS prescriptions in the survey with the total number of pharmacies and PBS prescriptions in Australia for each stratum. Volumes of non-subsidised drug use are calculated by multiplying the survey estimate by the weighting factor, which is assumed to apply equally to the subsidised and non-subsidised prescription volumes.
Combined database
A Departmental database combines the prescription estimates for the non-subsidised sector, under general co-payment and private prescriptions, based on the survey with the actual counts of those prescription categories submitted to the Department of Human Services for payment of a PBS/RPBS subsidy by the Government. A number of other Government subsidised programs and PBS programs subsidised through alternative supply arrangements are not included in the DUSC database. This includes the use of highly specialised drugs available for out-patients through public hospital pharmacies under Section 100 Highly Specialised Drugs Program of the National Health Act 1953, supply of drugs to remote area Aboriginal Health Services, Opioid Dependence Scheme, IVF/ GIFT Treatment and Botulinum Toxin Program. Subsidised programs operated by State Health Departments, including supply of drugs to public hospital in-patients, and the Herceptin Program for metastatic breast cancer are also excluded from the DUSC database. The combined dataset does contain information on highly specialised drugs and in-patient drugs provided through private hospitals as this information is collected by Medicare Australia. Following a range of pharmaceutical reforms a greater number of s100 Highly Specialised Drugs, and outpatient or discharge drugs supplied through public hospital will be included in the DUSC database in the future.
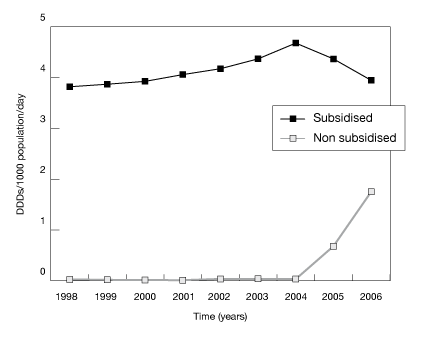
The advantages of the expanded database can be illustrated by using an example involving the drug fluoxetine. Previously, fluoxetine had a price per prescription, as a general benefit, above the patient co-payment and, as a consequence, a majority of community use was captured on the PBS/RPBS claims database. In 2004, a reduction in drug price and a small increase in the general patient co-payment in line with the Consumer Price Index (CPI) contributed to the price of fluoxetine falling below the general patient co-payment. Changes in the capturing of fluoxetine data from the Medicare claims database to the Pharmacy Guild Survey database can be seen in figure A. The combined database therefore enables the continuation of utilisation estimates for drugs not subsidised through the PBS/RPBS and provides a more comprehensive outlook on drug use within Australia.
Figure A shows the time trends for dispensing of fluoxetine, by the subsidised and non-subsidised components.
Quantities within the PBS Schedule are designed to provide a normal course of treatment for acute conditions, and a month’s treatment at usual doses for chronic conditions.
A pattern involving PBS drug utilisation that shows a higher level of usage leading up to the end of a year has been previously reported4. This peak is due to the safety net provisions introduced into the PBS in November 1986. These provisions were introduced to financially support patients with multiple medical conditions who genuinely need a number of medicines. Once the out-of-pocket threshold safety net level is reached, prescriptions on the scheme are either free, or available at a greatly reduced co-payment amount. The safety net period is the calendar year, and the highs and lows are due to stockpiling of medication once the safety net level is reached.
The stockpiling of medication has public health, waste and cost implications. Large quantities of potent medicines in the home can be a hazard for other family members, may exceed their expiry date, and has the potential for patient confusion if the dosage or the need for a particular medication is subsequently reviewed by the doctor during this period.
From 1 November 1994 the National Health (Pharmaceutical Benefits) Regulations have been amended to increase the period for redispensing chronically used drugs (i.e. those with 5 or more repeats) to a period of no less than 20 days. Exceptions are determined by the PBAC and include eye drops, which tend to be used at a higher rate than other medications. The redispensing period here was amended to four rather than the previous three days.
The pharmacist has the discretion to supply earlier than the statutory period if the circumstances warrant e.g. medicine lost or prescribed dosage requires more frequent dispensing of repeats.
Analyses of the effect of the 20 day resupply rule suggest a smoothing out of the highs and lows traditionally seen at the end and start of a safety net year respectively, although the total number of prescriptions dispensed has remained reasonably constant.
Figure A: Community utilisation of fluoxetine
ADVERSE DRUG REACTIONS REPORTING IN AUSTRALIA
The Therapeutic Goods Administration (TGA) is responsible for regulating medicines in Australia, including monitoring the ongoing safety of medicines once they have been included on the Australian Register of Therapeutic Goods (ARTG).
The TGA’s reporting system for adverse drug reactions began in the late 1960’s with the computerised database dating back to November 1972. At the end of 2010 there were approximately 233,300 reports of suspected adverse drug reactions in the database.
Figure B: Origin of adverse drug reaction reports received by the TGA (2005–2010)
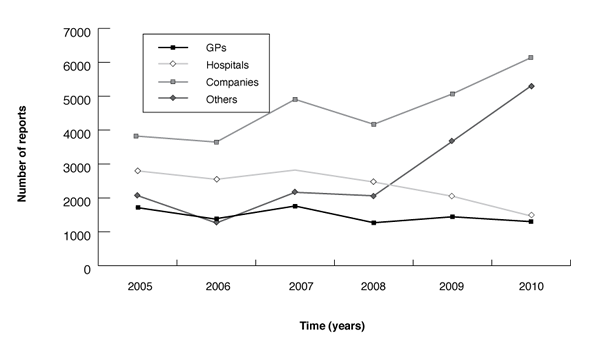
In 2010 the TGA received approximately 14,200 reports with 43% from pharmaceutical companies, 10% from hospitals, 9% from general practitioners and the remainder from other sources including State and Territory Health Departments, members of the public, community pharmacists and specialists (Figure B). On average, in 2010, the TGA received 1,184 reports per month. The TGA encourages practitioners to report suspected adverse reactions directly rather than through the manufacturer to make communication simpler. The increase in report numbers in 2010, particularly from ‘other’ sources and companies, is largely due to reports of adverse events following vaccination with seasonal trivalent influenza vaccines. Similarly, the increase in 2009 was largely due to reports of adverse events following vaccination with pandemic (H1N1) influenza vaccine.
How adverse drug reaction reports are processed and used
Reports are assessed by the Office of Product Review (OPR) within the TGA. This involves checking the report for the presence of ‘minimum’ details, i.e. an individual patient, an adverse reaction, at least one (suspected) drug, and, preferably, an identifiable reporting health professional. The specific reaction terms are identified along with the suspected, interacting or ‘other’ drugs and these are entered into the database.
The TGA applies a causality rating for the reaction(s) and in some cases requests further clinical or laboratory information from the reporter to allow causality to be assessed. Medical officers review serious reports and staff in OPR regularly analyse reporting data to identify potential safety signals.
Reports are forwarded to the Uppsala Monitoring Centre in Sweden which administers the WHO Collaborating Centre for International Drug Monitoring. This global database began in 1968 as a pilot program involving 10 nations, including Australia, and now receives reports from over 80 nations with over 5 million reports in the database.
How to report adverse drug reactions
The TGA encourages the reporting of all suspected adverse reactions to any medicine available in Australia, including prescription medicines, vaccines and over the counter and complementary medicines. The reporting of seemingly insignificant or common adverse reactions can contribute to the TGA’s investigation of a potential safety signal.
The TGA particularly requests reports of:
- All suspected reactions to new medicines
- All suspected medicines interactions
- Unexpected reactions (ie, those reactions that are not described in the Product Information)
- Suspected reactions causing:
- death
- admission to hospital or prolongation of hospitalisation
- increased investigations or treatment
- birth defects
Reports of suspected adverse drug reactions can be made:
- online at http://www.tga.gov.au by following the link to ‘Report a Problem’
- using a ‘Blue Card’ available from the TGA’s Office of Product Review
(1800 044 114) or downloaded from the TGA website at http://www.tga.gov.au/safety/problem-medicines-forms-bluecard.htm
Expert advisory committee
In January 2010, a new statutory expert advisory committee called the Advisory Committee on the Safety of Medicines (ACSOM) was established.
The major roles for ACSOM are to provide expert advice to the TGA about safety issues under investigation and the quality and appropriateness of Risk Management Plans (RMPs). RMPs have been required with applications for registration of ‘high risk’ medicines such as new chemical entities from April 2009 and are designed to characterise and pro-actively manage risks relating to a medicine over its entire life cycle. ACSOM also provides advice to the TGA on other matters related to pharmacovigilance, including the detection, assessment, understanding and prevention of adverse effects.
Medicines Safety Update
In 2010 Medicines Safety Update replaced the Australian Adverse Drug Reactions Bulletin. The Medicines Safety Update is published 6 times a year in Australian Prescriber, and also on the TGA website.
Articles published in Medicines Safety Update in 2010 included:
- A new era of medicines safety monitoring and communication of benefit—risk information at the TGA
- Enhanced postmarket risk management—Risk Management Plans
- ACSOM—a new expert advisory medicines safety committee
- Improved access to prescribing and consumer information
- Safety of fish oil and omega-3 fatty acids
- AUST R and AUST L numbers—why are they important?
- Sibutramine
- Drug-induced pancreatitis and exenatide (Byetta)
- Varenicline (Champix): an update
- Australian experience with non-adjuvant H1N1 vaccine (Panvax and Panvax Junior)
- Cholinesterase inhibitors and syncope
- Statins, macrolides and rhabdomyolysis
- Uterine perforation with levonorgestrel-releasing intrauterine system (Mirena)
- Rivaroxaban (Xarelto)—an overview of adverse event reports
- Lamotrigine and serious skin reactions
- Serotonin syndrome: a reminder
- Drug-induced acute akathisia
- Unintended pregnancy due to interaction between etonogestrel implant (Implanon) and carbamazepine
The Drugs of Current Interest Scheme
The aim of the ‘Drugs of Current Interest’ (DOCI) scheme was to undertake enhanced and focused pharmacovigilance for new drugs that may have received widespread use and for which the TGA was interested in obtaining a comprehensive post-market safety profile.
‘Drugs of Current Interest’ used to be selected by the Adverse Drug Reactions Advisory Committee (ADRAC). The DOCI list was published on the front page of the Australian Adverse Drug Reactions Bulletin and served as an indicator to readers of the drugs for which the TGA particularly requested adverse drug reaction reports.
ADRAC ceased to exist at the end of 2009, and a new committee—the Advisory Committee on the Safety of Medicines—was established to provide expert advice to the TGA on drug safety matters. Also in 2009, the TGA began to require sponsors of new medicines to submit a ‘Risk Management Plan’ (RMP) outlining the activities that they would undertake to monitor and communicate with health professionals and the public about the risks of medicines. RMPs are evaluated by the Office of Product Review as part of the evaluation of applications for registration. In addition, sponsors are required to submit to the TGA ‘Periodic Safety Update Reports’ (PSURs) annually for at least the first 3 years after the drug is approved. PSURs include summaries of spontaneous reporting as well as other sources of safety information for the drug. Late 2009 also saw the introduction of Australian Public Assessment Reports for Prescription Medicines (AusPARs). AusPARs provide information about the evaluation of a prescription medicine and the considerations that led the TGA to approve or not approve an application. AusPARs incorporate the Product Information (PI) approved at the time of releasing the AusPAR. The TGA website also contains a PI/Consumer Medicines Information (CMI) search facility which provides up-to-date copies of the PI/CMI.
In the light of these changes, and the overlap between RMPs, PSURs and the DOCI scheme, the DOCI scheme is no longer maintained by the TGA; although the TGA is continuing to consider ways to focus reporting on new medicines or those with extensions of their indication into new populations. The TGA continues to monitor the safety of all medicines registered in Australia and encourage health professionals to report all suspected adverse drug reactions to the TGA.
THE HIGHLY SPECIALISED DRUGS PROGRAM
Overview
The Australian Government provides funding for certain specialised medications under the Highly Specialised Drugs Program. Highly specialised drugs (HSDs) are medicines for the treatment of chronic conditions which, because of their clinical use or other special features, are restricted to supply through public and private hospitals having access to appropriate specialist facilities. To prescribe these drugs as pharmaceutical benefit items, medical practitioners are required to be affiliated with these specialist hospital units. A general practitioner or non-specialist hospital doctor may prescribe HSDs to provide maintenance therapy under the guidance of the treating specialist.
Subsidy for drugs under this program commences after approval has been given by the Australian Government and after the States and Territories agree to the administrative arrangements. For HSDs prescribed through private hospitals, claiming and approval of authority prescriptions is administered by Medicare Australia. For HSDs prescribed through public hospitals, access to the program is administered by the State/Territory health departments. From July, 2009 Medicare Australia commenced collection of information on drugs supplied through public hospitals. From July 2010, Medicare Australia commenced receiving information on HSDs through public hospitals at an individual script level rather than summarised at itemcode level. Public hospitals are transitioning to these new claiming arrangements and it is expected that by the end of December 2012, this transition will be completed. This will enable the incorporation of public hospital HSDs data into Tables 1 and 2.
The Australian Government provides funding for a HSD to be supplied to community based patients not in-patients of public hospitals; i.e. persons who are day-admitted patients, outpatients and patients upon discharge.
Highly Specialised Drugs Working Party
The Highly Specialised Drugs Working Party (HSDWP) was established by the Australian Health Ministers’ Advisory Council in 1991. It consists of representatives from the Health Departments of each of the States and Territories, the Australian Private Hospitals Association, and the Australian Government as chair. The main purpose of the HSDWP is to identify, and refer for consideration by the PBAC, those drugs which meet the selection criteria for HSDs.
Criteria for selection of Highly Specialised Drugs
- Drugs recommended for inclusion in the program must satisfy the following criteria:
- Ongoing specialised medical supervision required.
- Treatment of longer term medical conditions, not episodes of in-patient treatment or treatment of acute conditions.
- Drug highly specialised and an identifiable patient target group.
- Subject to marketing approval by the TGA and specific therapeutic indications covered by the terms of the marketing letter from TGA.
- High unit cost.
Table A: Highly Specialised Drugs—National Usage and Patient Report in
Public Hospitals for the period January 2010 to December 2010
| Drug Name | Total Cost ($Aus) | Pack Numbers |
|---|---|---|
| ABACAVIR | $1,611,856 | 5,892 |
| ABACAVIR with LAMIVUDINE | $17,936,619 | 31,803 |
| ABACAVIR with LAMIVUDINE and ZIDOVUDINE | $1,633,156 | 1,917 |
| ABATACEPT | $2,450,352 | 4,794 |
| ADALIMUMAB | $41,493 | 25 |
| ADEFOVIR DIPIVOXIL | $5,075,347 | 8,121 |
| AMBRISENTAN | $982,180 | 242 |
| APOMORPHINE HYDROCHLORIDE | $3,042,021 | 24,805 |
| ATAZANAVIR | $17,868,405 | 32,447 |
| AZITHROMYCIN | $74,076 | 1,260 |
| BACLOFEN | $1,092,472 | 7,363 |
| BOSENTAN MONOHYDRATE | $6,802,930 | 1,678 |
| CIDOFOVIR | $5,400 | 6 |
| CINACALCET | $6,183,469 | 14,224 |
| CLARITHROMYCIN | $108,579 | 1,798 |
| CLOZAPINE | $41,593,822 | 153,605 |
| CYCLOSPORIN | $10,195,094 | 124,970 |
| DARBEPOETIN ALFA | $58,373,983 | 91,687 |
| DARUNAVIR | $8,415,496 | 7,939 |
| DEFERASIROX | $11,476,565 | 15,638 |
| DEFERIPRONE | $657,187 | 1,497 |
| DELAVIRDINE MESYLATE | $9,777 | 36 |
| DESFERRIOXAMINE MESYLATE | $862,496 | 16,408 |
| DIDANOSINE | $474,403 | 1,721 |
| DISODIUM PAMIDRONATE | $1,388,463 | 5,136 |
| DORNASE ALFA | $9,579,216 | 8,118 |
| DOXORUBICIN HYDROCHLORIDE, PEGYLATED LIPOSOMAL | $154,878 | 249 |
| EFAVIRENZ | $6,396,217 | 16,479 |
| EMTRICITABINE | $207,620 | 736 |
| ENFUVIRTIDE | $800,774 | 362 |
| ENTECAVIR MONOHYDRATE | $10,607,842 | 25,159 |
| EPOETIN ALFA | $23,675,542 | 33,043 |
| EPOETIN BETA | $10,870,738 | 15,845 |
| EPOPROSTENOL SODIUM | $382,091 | 5,086 |
| ETANERCEPT | $475,841 | 511 |
| ETRAVIRINE | $3,022,669 | 4,903 |
| EVEROLIMUS | $5,075,931 | 8,958 |
| FILGRASTIM | $13,823,644 | 7,820 |
| FOSAMPRENAVIR | $660,335 | 1,757 |
| FOSCARNET SODIUM | $1,452 | 4 |
| GANCICLOVIR | $110,345 | 394 |
| IBANDRONIC ACID | $403,508 | 1,182 |
| ILOPROST TROMETAMOL | $770,722 | 749 |
| INDINAVIR | $100,506 | 221 |
| INFLIXIMAB | $17,163,089 | 22,707 |
| INTERFERON ALFA-2a | $40,664 | 979 |
| INTERFERON ALFA-2b | $679,107 | 1,730 |
| INTERFERON GAMMA-1b | $420,963 | 309 |
| LAMIVUDINE | $4,410,185 | 23,189 |
| LAMIVUDINE with ZIDOVUDINE | $4,457,955 | 7,705 |
| LANREOTIDE ACETATE | $5,454,655 | 3,003 |
| LANTHANUM | $1,226,324 | 3,423 |
| LENALIDOMIDE | $15,915,797 | 2,443 |
| LENOGRASTIM | $169,152 | 213 |
| LOPINAVIR with RITONAVIR | $11,994,282 | 17,949 |
| MARAVIROC | $700,077 | 892 |
| METHOXY POLYETHYLENE GLYCOL-EPOETIN BETA | $1,742,745 | 2,652 |
| MYCOPHENOLATE MOFETIL | $16,389,220 | 88,349 |
| MYCOPHENOLATE SODIUM | $2,584,289 | 6,244 |
| NATALIZUMAB | $22,107,331 | 10,845 |
| NEVIRAPINE | $8,824,190 | 32,582 |
| OCTREOTIDE | $12,482,205 | 10,647 |
| PEGFILGRASTIM | $46,391,881 | 24,099 |
| PEGINTERFERON ALFA-2a | $2,265,694 | 1,693 |
| PEGINTERFERON ALFA-2b | $91,172 | 37 |
| RALTEGRAVIR | $13,855,372 | 15,219 |
| RIBAVIRIN and PEGINTERFERON ALFA-2a | $31,300,578 | 19,173 |
| RIBAVIRIN and PEGINTERFERON ALFA-2b | $13,470,571 | 7,227 |
| RIFABUTIN | $52,598 | 358 |
| RITONAVIR | $1,762,571 | 28,294 |
| RITUXIMAB | $2,649,895 | 1,167 |
| SAQUINAVIR | $534,281 | 1,057 |
| SEVELAMER HYDROCHLORIDE | $3,082,224 | 9,943 |
| SILDENAFIL CITRATE | $230,241 | 245 |
| SIROLIMUS | $2,711,023 | 3,323 |
| SITAXENTAN SODIUM | $555,672 | 201 |
| STAVUDINE | $299,393 | 732 |
| TACROLIMUS | $21,749,627 | 56,745 |
| TELBIVUDINE | $23,834 | 95 |
| TENOFOVIR | $8,706,826 | 18,018 |
| TENOFOVIR with EMTRICITABINE | $38,649,399 | 50,515 |
| TENOFOVIR with EMTRICITABINE and EFAVIRENZ | $29,841,961 | 24,506 |
| THALIDOMIDE | $8,164,297 | 19,206 |
| TIPRANAVIR | $199,281 | 211 |
| TOCILIZUMAB | $587,895 | 970 |
| VALACICLOVIR | $333,969 | 789 |
| VALGANCICLOVIR HYDROCHLORIDE | $11,380,533 | 5,164 |
| ZIDOVUDINE | $403,729 | 1,825 |
| ZOLEDRONIC ACID | $12,371,867 | 29,851 |
| Grand Total | $663,904,128 | 1,243,113 |
Supply of pharmaceutical benefits to remote area Aboriginal Health Services (AHSs) under Section 100 of the National Health Act
The S100 Supply Arrangements for Remote Area Aboriginal Health Services (AHSs) improve access to the PBS for clients of remote area AHSs under Section 100 of the National Health Act 1953.
Under these arrangements, clients of participating AHSs are able to receive PBS medicines directly from the AHS at the point of consultation, without the need for a normal prescription form, and without charge.
The eligibility criteria for participation in the program are given below.
Eligibility criteria
- The health service must have a primary function of meeting the health care needs of Aboriginal and Torres Strait Islander peoples.
- The clinic or other health care facility operated by the AHS from which pharmaceuticals are supplied to patients must be in a remote zone as defined in the Rural, Remote and Metropolitan Areas Classification 1991 Census Edition.
- The AHS must not be a party to an arrangement, such as a coordinated care trial, for which funds from the PBS have already been provided.
- The AHS must employ or be in a contractual relationship with health professionals who are suitably qualified under relevant State/Territory legislation to supply all medications covered by the Section 100 arrangements and undertake that all supply of benefit items will be under the direction of such qualified persons.
The clinic or other health care facility operated by the AHS from which pharmaceuticals are supplied must have storage facilities that will:
- prevent access by unauthorised persons;
- maintain the quality (eg chemical and biological stability and sterility) of the pharmaceutical; and
- comply with any special conditions specified by the manufacturer of the pharmaceutical.
Expenditure
There are 172 AHSs participating in the program from the Northern Territory, Queensland, Western Australia, South Australia, New South Wales and Tasmania. PBS expenditure via these arrangements for the calendar year of 2010 (including GST) was $45 million.
HEALTH EXPENDITURE TRENDS
Table B (i): Total expenditure on pharmaceuticals and other medical non-durables as % total expenditure on health, TEH
| 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Australia | 13.8 | 14.8 | 15.1 | 14.5 | 15.0 | 14.8 | 14.3 | 14.3 | 14.3 | 14.6 | n.a. |
| Canada | 15.6 | 15.9 | 16.2 | 16.6 | 17.0 | 17.3 | 17.2 | 17.4 | 17.2 | 17.0 | 17.0 |
| France | 16.0 | 16.5 | 16.9 | 16.8 | 16.7 | 16.8 | 16.7 | 16.3 | 16.4 | 16.3 | 16.1 |
| Germany | 13.5 | 13.6 | 14.2 | 14.4 | 14.4 | 13.8 | 15.0 | 14.7 | 15.0 | 15.0 | 14.9 |
| New Zealand | n.a. | n.a. | n.a. | n.a. | n.a. | 10.4 | 10.3 | 10.9 | 10.1 | 9.4 | 9.3 |
| United Kingdom | n.a. | 14.2 | 13.9 | 13.6 | 13.5 | 13.3 | 12.8 | 12.3 | 12.2 | 11.6 | n.a. |
| United States | 10.7 | 11.3 | 11.7 | 11.9 | 12.1 | 12.2 | 12.1 | 12.2 | 12.1 | 11.9 | 12.0 |
Table B (ii): Total expenditure on pharmaceuticals & other medical non-durables per capita, US$ purchasing power parity
| 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Australia | 290 | 336 | 361 | 370 | 400 | 425 | 426 | 453 | 480 | 503 | n.a. |
| Canada | 376 | 401 | 442 | 479 | 519 | 553 | 591 | 636 | 661 | 684 | 744 |
| France | 384 | 421 | 462 | 492 | 500 | 525 | 553 | 570 | 605 | 622 | 640 |
| Germany | 348 | 362 | 397 | 422 | 445 | 439 | 505 | 526 | 559 | 594 | 628 |
| New Zealand | n.a. | n.a. | n.a. | n.a. | n.a. | 213 | 227 | 270 | 254 | 261 | 276 |
| United Kingdom | n.a. | 260 | 278 | 296 | 313 | 338 | 350 | 368 | 371 | 381 | n.a. |
| United States | 485 | 540 | 600 | 665 | 724 | 772 | 808 | 866 | 900 | 919 | 956 |
Table B (iii): Total expenditure on health as % gross domestic product
| 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Australia | 7.8 | 8.0 | 8.1 | 8.4 | 8.3 | 8.5 | 8.4 | 8.5 | 8.5 | 8.7 | n.a. |
| Canada | 8.9 | 8.8 | 9.3 | 9.6 | 9.8 | 9.8 | 9.8 | 10.0 | 10.0 | 10.3 | 11.4 |
| France | 10.1 | 10.1 | 10.2 | 10.5 | 10.9 | 11.0 | 11.1 | 11.0 | 11.0 | 11.1 | 11.8 |
| Germany | 10.3 | 10.3 | 10.4 | 10.6 | 10.8 | 10.6 | 10.7 | 10.6 | 10.5 | 10.7 | 11.6 |
| New Zealand | 7.5 | 7.6 | 7.7 | 8.0 | 7.9 | 8.3 | 8.7 | 9.1 | 8.8 | 9.6 | 10.3 |
| United Kingdom | 6.9 | 7.0 | 7.2 | 7.6 | 7.8 /td> | 8.0 | 8.2 | 8.5 | 8.4 | 8.8 | 9.8 |
| United States | 13.6 | 13.7 | 14.3 | 15.2 | 15.7 | 15.7 | 15.7 | 15.8 | 16.0 | 16.4 | 17.4 |
Legend
n.a.: Not available (data not provided to the Organisation for Economic Co-operation and Development (OECD) by these countries for these years)
Sources: OECD Health Data 2011—Version June 2011
(a) ‘Pharmaceuticals’ defined
The OECD definition of pharmaceuticals has been used, as described in its System of Health Accounts(SHA). The OECD defines pharmaceuticals as ‘pharmaceuticals and other medical non-durables dispensed to out-patients’, which comprises prescription medicines, over-the-counter medicines and other medical non-durables. Pharmaceuticals dispensed to, or used by admitted patients in hospital are not included.
Broadly speaking, these include medicinal preparations, branded and generic medicines, drugs, patent medicines, serums and vaccines, vitamins and minerals, and oral contraceptives and a wide range of medical non-durable goods, which are either single use items, for example bandaids and condoms, or have limited re-usage, for example, bandages.
Prescribed medicines are medicines exclusively sold to customers with a medical voucher, irrespective of whether it is covered by public or private funding and include branded and generic products. In the SHA, this includes the full price with a breakdown for cost-sharing.
Expenditure by private households or over-the-counter medicines (OTC medicines) is included in pharmaceutical expenditure.
Other medical non-durables comprise items such as bandages, elastic stockings, incontinence articles, condoms and other mechanical contraceptive devices.
Pharmaceutical expenditure is also reported in Health Expenditure Australia 2009–10. In this report, the Australian Institute of Health and Welfare (AIHW) splits medication expenditure into two categories, ‘benefit paid pharmaceuticals’ and ‘all other medications’.
‘Benefit paid pharmaceuticals’ are pharmaceuticals in the Pharmaceutical Benefits Scheme (PBS) and the Repatriation Pharmaceutical Benefits Scheme (RPBS) for which the Australian Government paid a benefit.
‘All other medications’ are medications for which no PBS or RPBS benefit was paid and includes the following components, which are reported separately:
- pharmaceuticals listed in the PBS or RPBS, where the total costs are equal to, or
less than, the statutory patient contribution for the class of patient concerned (‘under
co–payment’ drugs); - medicines dispensed through private prescriptions for items not listed in the PBS or RPBS or which do not meet PBS criteria for dispensing through the PBS; and
- over–the–counter medicines such as aspirin, cough and cold medicines, vitamins and minerals, some herbal and other complementary medicines and medical non-durable goods, as listed above.
Under the AIHW definitions used in Health expenditure Australia, highly specialised drugs are included as part of hospital expenditure, not as part of medications expenditure. Under the OECD definition, highly specialised drugs are included in ‘Pharmaceutical’ expenditure.
(b) Health expenditure recorded here is according to the OECD definition and excludes health research expenditure.
DRUG UTILISATION TRENDS
Anatomical Therapeutic Chemical (ATC) classification index with Defined Daily Doses (DDDs) 2011 is used in all statistics published in this edition (refer to WHO collaborating Centre for Drug Statistics Methodology, ATC classification index with DDDs 2011).
Listed below are the prescription counts for 2008, 2009 and 2010 by ATC anatomical main group. The data from the two sources are enumerated separately. Table C (i) shows subsidised prescriptions (PBS/RPBS) and Table C (ii) shows the estimate of non-subsidised prescriptions (Survey).
Tables C: Prescription numbers by ATC groups
Table C (i): Subsidised prescriptions (PBS/RPBS)
| ATC Group | 2008 | 2009 | 2010 |
|---|---|---|---|
| (A) Alimentary Tract | 27,494,955 | 28,400,464 | 28,929,033 |
| (B) Blood and blood forming | 8,349,546 | 8,640,003 | 8,959,158 |
| (C) Cardiovascular system | 65,586,480 | 67,302,312 | 69,490,439 |
| (D) Dermatologicals | 2,971,054 | 2,980,367 | 3,044,278 |
| (G) Genitourinary system | 3,530,906 | 3,172,962 | 3,016,515 |
| (H) Hormonal preparations | 2,844,392 | 2,906,146 | 3,027,611 |
| (J) Antiinfectives | 13,382,468 | 13,546,581 | 13,601,137 |
| (L) Antineoplastic | 1,908,120 | 2,098,520 | 2,282,506 |
| (M) Musculo-skeletal | 9,667,741 | 9,262,036 | 9,042,582 |
| (N) Nervous system | 37,260,541 | 38,838,056 | 41,098,982 |
| (P) Antiparasitic products | 552,215 | 583,591 | 619,501 |
| (R) Respiratory system | 10,462,951 | 10,544,777 | 10,922,206 |
| (S) Sensory Organs | 8,619,154 | 8,718,735 | 8,823,748 |
| (V) Various | 665,539 | 646,443 | 627,491 |
| Other | 193,789 | 209,959 | 234,102 |
| Total | 193,489,851 | 197,850,952 | 203,719,289 |
Table C (ii): Estimated non-subsidised prescriptions (Survey)
| ATC Group | 2008 | 2009 | 2010 |
|---|---|---|---|
| (A) Alimentary Tract | 4,885,310 | 5,394,413 | 5,443,423 |
| (B) Blood and blood forming | 837,414 | 826,678 | 799,144 |
| (C) Cardiovascular system | 12,799,049 | 13,069,382 | 12,749,658 |
| (D) Dermatologicals | 3,161,476 | 2,998,874 | 3,090,690 |
| (G) Genitourinary system | 7,409,095 | 7,319,091 | 7,241,951 |
| (H) Hormonal preparations | 1,607,771 | 1,600,906 | 1,673,213 |
| (J) Antiinfectives | 15,935,985 | 15,530,330 | 15,262,828 |
| (L) Antineoplastic | 113,038 | 118,612 | 134,160 |
| (M) Musculo-skeletal | 3,109,691 | 3,168,661 | 3,088,987 |
| (N) Nervous system | 12,128,260 | 12,106,849 | 12,179,569 |
| (P) Antiparasitic products | 726,220 | 691,724 | 671,856 |
| (R) Respiratory system | 2,759,011 | 2,696,789 | 2,648,049 |
| (S) Sensory Organs | 2,545,218 | 2,400,492 | 2,294,943 |
| (V) Various | 18,600 | 19,842 | 21,220 |
| Other | 243,830 | 220,046 | 232,186 |
| Total | 68,279,968 | 68,162,689 | 67,531,877 |
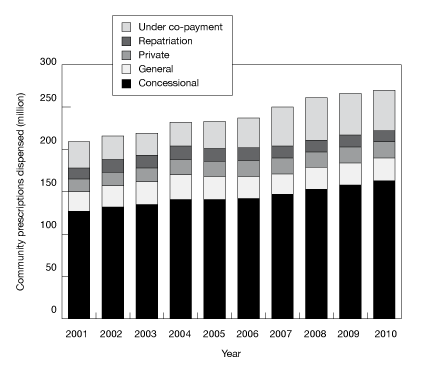
Estimated changes from 2001 to 2010 in the number of prescriptions dispensed under the PBS (concession and general), RPBS, under co-payment and private categories, are presented in Figure C.
Figure C: Number of prescriptions by type of service
Most commonly used drugs in the Australian community for 2010
Table D shows the top 10 drugs dispensed in the Australian community by DDDs/1000 population/day, which adjusts for the quantity dispensed per prescription. This DDDs/1000/day information shows both the subsidised (PBS/RPBS) and non-subsidised (Guild survey) components, as well as total community use. Changes and alterations from the previous years are also shown.
Table D: Top 10 drugs by defined daily dose/thousand population/day, 2010
(including the contribution of constituents of combination products)
| Drug | PBS/RPBS | Guild Survey | Total |
|---|---|---|---|
| 1. ATORVASTATIN | 82.754 | 0.117 | 82.871 |
| 2. IRBESARTAN | 33.606 | 10.949 | 44.556 |
| 3. PERINDOPRIL | 29.334 | 11.058 | 40.392 |
| 4. RAMIPRIL | 26.902 | 9.568 | 36.471 |
| 5. ROSUVASTATIN | 29.447 | 0.056 | 29.503 |
| 6. CANDESARTAN | 23.822 | 5.630 | 29.453 |
| 7. PARACETAMOL | 25.804 | 2.368 | 28.172 |
| 8. SIMVASTATIN | 24.714 | 0.733 | 25.447 |
| 9. AMLODIPINE | 18.768 | 3.853 | 22.620 |
| 10. ESOMEPRAZOLE | 22.151 | 0.096 | 22.247 |
Changes from 2009:
|
UP: |
Rosuvastatin (8 to 5) Amlodipine (10 to 9) |
|
DOWN: |
Candesartan (5 to 6) Simvastatin (6 to 8) Esomeprazole (9 to 10) |
The top 10 drugs dispensed in the Australian community in 2010, ranked by prescription count, are shown in table E. Table F ranks the 2010 top 10 drugs by total cost to Australia, i.e. subsidised prescriptions only (total cost is the sum of patient contribution and cost to Government).
Table E: Top 10 drugs by prescription counts, 2010
| Drug | PBS/RPBS | Guild Survey | Total Community use |
|---|---|---|---|
| 1. ATORVASTATIN | 11,081,110 | 18,200 | 11,099,310 |
| 2. ESOMEPRAZOLE | 6,491,038 | 27,673 | 6,518,711 |
| 3. AMOXYCILLIN | 2,561,282 | 3,766,873 | 6,328,155 |
| 4. PERINDOPRIL | 4,034,112 | 1,512,680 | 5,546,792 |
| 5. ROSUVASTATIN | 5,411,982 | 10,518 | 5,422,500 |
| 6. PARACETAMOL | 4,617,367 | 246,799 | 4,864,166 |
| 7. CEFALEXIN | 2,646,909 | 2,210,497 | 4,857,406 |
| 8. SIMVASTATIN | 4,434,394 | 316,944 | 4,751,338 |
| 9. METFORMIN HYDROCHLORIDE | 3,422,482 | 1,110,941 | 4,533,423 |
| 10. PANTOPRAZOLE | 3,646,665 | 635,708 | 4,282,373 |
Changes from 2009:
| UP |
Esomeprazole (3 to 2) Paracetamol (8 to 6) |
| DOWN: |
Amoxycillin (2 to 3) Cefalexin (6 to 7) Simvastatin (5 to 8) Metformin Hydrochloride (7 to 9) |
| IN: |
Rosuvastatin (11 to 5) Pantoprazole (13 to 10) |
| OUT: |
Irbesartan (9 to 11) Atenolol (10 to 13) |
Table F: Top 10 PBS/RPBS drugs by total cost to Australia, 2010
| Drug | PBS/RPBS DDD/1000 POP/DAY |
PBS/RPBS Scripts | Total Cost |
|---|---|---|---|
| 1. ATORVASTATIN | 82.754 | 11,081,110 | 779,565,949 |
| 2. ROSUVASTATIN | 29.447 | 5,411,982 | 396,510,559 |
| 3. RANIBIZUMAB | 131,436 | 278,654,131 | |
| 4. ESOMEPRAZOLE | 22.151 | 6,491,038 | 269,267,266 |
| 5. CLOPIDOGREL | 10.935 | 2,907,670 | 214,582,857 |
| 6. SALMETEROL and FLUTICASONE | 3,023,294 | 213,950,711 | |
| 7. SIMVASTATIN | 24.714 | 4,434,394 | 182,154,198 |
| 8. OLANZAPINE | 3.013 | 949,384 | 169,938,409 |
| 9. ADALIMUMAB | 0.320 | 93,078 | 167,311,778 |
| 10. VENLAFAXINE | 12.452 | 2,667,247 | 143,866,900 |
No information on cost for private and under co-payment prescriptions is available.
Changes from 2009:
|
UP: |
Ranibizumab (7 to 3) |
|
DOWN: |
Esomeprazole (3 to 4) Clopidogrel (4 to 5) Salmeterol and Fluticasone (5 to 6) Simvastatin (6 to 7) Venlafaxine (9 to 10) |
|
IN: |
Adalimumab (11 to 9) |
|
OUT: |
Pantoprazole (10 to 12) |
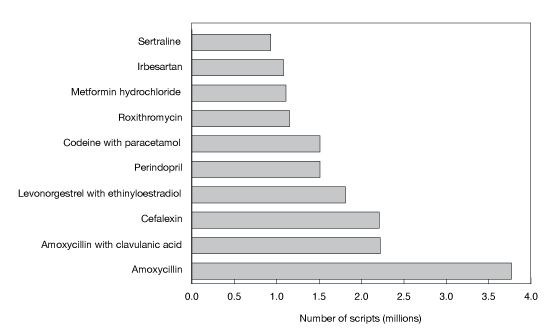
Figure D shows the top 10 subsidised drugs dispensed in 2010.
Figure D: Top 10 subsidised drugs dispensed in 2010
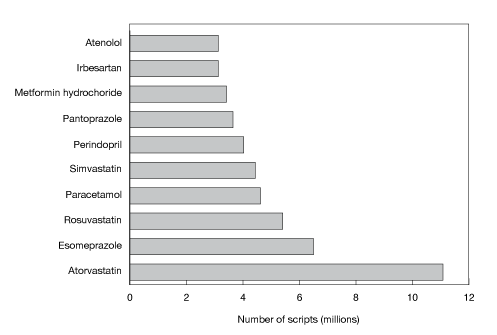
Figure E represents the top 10 non-subsidised drugs for 2010.
Figure E: Top 10 non-subsidised drugs dispensed in 2010
TABLES IN THE AUSTRALIAN STATISTICS ON MEDICINES
The data are presented in two major tables. Table 1 provides an estimate of the 2010 community (i.e. subsidised and non-subsidised) prescription numbers, together with the costs for PBS-listed drugs, which include an estimate of the cost of under co-payment PBS prescriptions. Cost information on the dispensing of private prescriptions is not available. The defined daily dose (DDD), where available, is also included for the drugs covered in the report.
Table 2 includes community prescription drug use, in DDDs/1000 population/day, for the years 2008, 2009 and 2010. In this edition, DDDs/1000 population/day for combination products is also reported in terms of DDDs of each constituent. One main advantage of reporting combinations as if they were administered as two or more single component products is that total DDDs remain constant as patients switch to combination products, if the amounts of constituent drugs consumed by patients remain the same.
Note that not all combination products are included in table 2. Combination drugs will only be reported in terms of DDD of each constituent where:
- the constituent drugs were available as plain drugs on the PBS;
- the combination was a frequently prescribed form;
- the constituent drugs had independent actions; and
- the WHO Defined Daily Dose was consistent across the various formulations of the constituent drugs.
Anatomical Therapeutic Chemical (ATC) classification index with Defined Daily Doses (DDDs) 2011 is used in statistics published in this edition (refer to WHO collaborating Centre for Drug Statistics Methodology, ATC classification index with DDDs 2011).
References
- Edmonds DJ, Dumbrell DM, Primrose JG, McManus P, Birkett DJ, Demirian V. Development of an Australian drug utilisation database: a report from the Drug Utilisation Subcommittee of the Pharmaceutical Benefits Advisory Committee, PharmacoEconomics 1993; 3(6): 427-432.
- Hurley SF, McNeil JJ. Drug-coding systems: why so many? Med J Aust 1989; 151: 308.
- Nordic Council on Medicines. Nordic Statistics on Medicines 1987–1989. NLN publication number 3, Uppsala, Sweden, 1990.
- McManus P. Drug utilisation (letter) Med J Aust 1993; 158: 724.
- WHO Collaborating Centre for Drug Statistics Methodology, ATC classification index with DDDs 2011. Oslo.
CAVEATS
It needs to be borne in mind that these utilisation data do not include a large proportion of public hospital drug usage, over the counter purchases (except for S3 Recordable), or the supply of highly specialised drugs to outpatients through public hospitals, under Section 100 of the National Health Act 1953. Some extemporaneously prepared items may also not be included.
Comments on classifications, omissions or errata appearing in this edition of the Australian Statistics on Medicines may be sent to:
Alicia Segrave
Secretary
Drug Utilisation Sub-Committee (DUSC)
Department of Health
GPO Box 9848
CANBERRA ACT 2601.
e-mail: DUSC@health.gov.au
GLOSSARY OF TERMS
| Actu | Actuated | Linct | Linctus |
| Adhes | Adhesive | Lin | Liniment |
| Admin | Administration | Liq | Liquid |
| Aero | Aerosol | Loz | Lozenge |
| Amp(s) | Ampoule(s) | Ltn | Lotion |
| Applic | Applicator | Metronid | Metronidazole |
| Aqu | Aqueous | Mixt | Mixture |
| Breth | Breath | Nas | Nasal |
| Calc | Calcium | Nebu | Nebuliser |
| Cap(s) | Capsule(s) | Not< | Not less than |
| Cart | Cartridge | Oint | Ointment |
| CD | Controlled delivery | Ophth | Ophthalmic |
| Chew | Chewable | Paed | Paediatric |
| Clean | Cleansing | Pdr | Powder |
| Coat | Coated | Pell(s) | Pellet(s) |
| Co | Compound | Pess | Pessary |
| Conc | Concentrated | Phos | Phosphorus |
| Cont | Contained | Pot | Potassium |
| CR | Controlled release | Prep | Preparation |
| Crm | Cream | Press | Pressurised |
| Crush | Crushable | Prot | Protective |
| D | Dose | Pst | Paste |
| Dev | Device | Reag | Reagent |
| Diag | Diagnostic | Rel | Release |
| Dil | Diluted | Requ | Required |
| Disp | Dispersable | Sach(s) | Sachet(s) |
| Dres | Dressing | SF | Sugar free |
| Drp | Drops | Sng | Single |
| Ds | Doses | Sod | Sodium |
| Dust | Dusting | Sol | Soluble |
| Efferv | Effervescent | Soln | Solution |
| Elx | Elixir | Solv | Solvent |
| Enter | Enteric | Spr | Spray |
| Emulsif | Emulsifying | Ster | Sterile |
| Equiv | Equivalent | Sulph | Sulphate |
| Extend | Extended | Suppl | Supplement |
| Ferr | Ferrous | Suppos | Suppository |
| Gran | Granules | Supres | Suppression |
| Inf | Infusion | Susp | Suspension |
| Inhal | Inhalation | Sust | Sustained |
| Inj(s) | Injection(s) | Syrp | Syrup |
| Inrt | Inert | Syrng | Syringe |
| Ins | Insert | Tab(s) | Tablet(s) |
| Intracav | Intracavernosal | Td | Transdermal |
| Intranas | Intranasal | Tinct | Tincture |
| Insuff | Insufflator | Top | Topical |
| Irrig | Irrigation | Unt(s) | Unit(s) |
| Jel | Jelly | wps | Wipes |
Weights and Measures
| cm | centimetre(s) |
| E | unit(s) |
| g | gram(s) |
| kg | kilogram(s) |
| iu | international unit |
| L | litre(s) |
| m | metre(s) |
| ME | million units |
| mm | millimetre(s) |
| mg | milligram(s) |
| mL | millilitre(s) |
| mmol | millimole |
| TE | thousand units |
| ug | micrograms(s) |
ATC & DDD Additions and Alterations
Alterations in ATC classification
| Drug/drug group | Previous ATC code | New ATC code |
|---|---|---|
| Levonorgestrel | G03AC03 | G03AD011) |
| Phentolamine | G04BE05 | V03AB36 |
| Artemether and Lumefantrine2) | P01BE52 | P01BF01 |
| Ephedrine | R03CA02 | C01CA263) |
1) Oral products (packages) only indicated for emergency contraception
2) New ATC 5th level name; changed from artemether, combinations (P01BE52)
3) Only parenteral formulations
Alterations in DDDs
| ATC code | ATC level name | Previous DDD | New DDD |
|---|---|---|---|
| A10BB09 | Gliclazide | 0.16 g O | 60 mg O |
| B02BD09 | Nonacog alfa | 1000 U P | 450 U P |
Alterations in ATC level classification
| ATC code | Previous ATC level name | New ATC level name |
|---|---|---|
| B03AB05 | Dextriferron | Ferric oxide polymaltose complexes |
| B03AC01 | Dextriferron | Ferric oxide polymaltose complexes |
| B03AC06 | Ferric oxide dextran complex | Ferric oxide dextran complexes |
| B03AD04 | Dextriferron | Ferric oxide polymaltose complexes |
| C01BG | Other class I antiarrhythmics | Other antiarrhythmics, class I and III |
| G03AD | Emergency contraceptives | |
| P01BE | Artemisinin and derivatives | Artemisinin and derivatives, plain |
| P01BF | Artemisinin and derivatives, combinations |
Allocation of new ATC codes
| New ATC Code | ATC level name |
|---|---|
| A02BD08 | Bismuth subcitrate, tetracycline and metronidazole |
| A10BH05 | Linagliptin |
| A16AB10 | Velaglucerase alfa |
| A16AB11 | Taliglucerase alfa |
| B01AC23 | Cilostazol |
| B01AC24 | Ticagrelor |
| B01AC56 | Acetylsalicylic acid and esomeprazole |
| C01BD07 | Dronedarone |
| C01BG11 | Vernakalant |
| C01CA25 | Amezinium metilsulfate |
| C05AA12 | Triamcinolone |
| C05CX02 | Naftazone |
| C07BB12 | Nebivolol and thiazides |
| C07FB07 | Bisoprolol and other antihypertensives |
| C08CA16 | Clevidipine |
| C09BB06 | Enalapril and nitrendipine |
| C09DB05 | Irbesartan and amlodipine |
| C09DX03 | Olmesartan medoxomil, amlodipine and hydrochlorothiazide |
| C09XA53 | Aliskiren and amlodipine |
| C09XA54 | Aliskiren, amlodipine and hydrochlorothiazide |
| C10AB11 | Choline fenofibrate |
| C10BX04 | Simvastatin, acetylsalicylic acid and ramipril |
| G03AA15 | Chlormadinone and estrogen |
| G03AB08 | Dienogest and estrogen |
| G03AD02 | Ulipristal |
| G03XC03 | Lasofoxifene |
| J01DI02 | Ceftaroline fosamil |
| J01MA21 | Sitafloxacin |
| L01DB10 | Amrubicin |
| L01DB11 | Pixantrone |
| L01XA05 | Polyplatillen |
| L01XE12 | Vandetanib |
| L01XE13 | Afatinib |
| L01XX40 | Omacetaxine mepesuccinate |
| L01XX41 | Eribulin |
| L03AB12 | Albinterferon alfa-2b |
| L04AA26 | Belimumab |
| L04AA27 | Fingolimod |
| L04AA28 | Belatacept |
| L04AC09 | Briakinumab |
| L04AD03 | Voclosporin |
| M01AE18 | Naproxcinod |
| M01AE52 | Naproxen and esomeprazole |
| M01AX26 | Avocado and soyabean oil, unsaponifiables |
| M02AA26 | Nimesulide |
| M02AX05 | Idrocilamide |
| M05BB05 | Alendronic acid, calcium and colecalciferol, sequential |
| M09AB02 | Collagenase clostridium histolyticum |
| M09AX02 | Chondrocytes, autologous1) |
| N03AX21 | Retigabine |
| N06BA12 | Lisdexamfetamine |
| N07XX07 | Fampridine |
| P01BF02 | Artesunate and mefloquine |
| P01BF03 | Artesunate and amodiaquine |
| P01BF04 | Artesunate, sulphamethopyrazine and pyrimethamine |
| P01BF05 | Artenimol and piperaquine |
| P01BF06 | Artesunate and pyronaridine |
| R05CB16 | Mannitol |
| R06AX29 | Bilastine |
| S01AX23 | Besifloxacin |
| V09IA07 | Technetium (99mTc) hynic-octreotide |
| V09IX08 | Fluoroethylcholine (18F) |
1) Chondrocytes previously classified in V03AX should be moved to the new code in M09AX02
Allocation of new DDDs
| ATC level name | ATC code | New DDD |
|---|---|---|
| A10BH03 | Saxagliptin | 5 mg O |
| A10BX07 | Liraglutide | 1.2 mg P |
| A11HA08 | Tocofersolan | 0.2 g1) O |
| B01AC23 | Cilostazol | 0.2 g O |
| B02AB03 | C1 inhibitor | 1.4 TU P |
| C01BD07 | Dronedarone | 0.8 g O |
| C01CA25 | Amezinium metilsulfate | 30 mg O |
| C03XA01 | Tolvaptan | 30 mg O |
| C10AB11 | Choline fenofibrate | 0.135 g2) O |
| G03AD02 | Ulipristal | 30 mg O |
| G03XC02 | Bazedoxifene | 20 mg O |
| G03XC03 | Lasofoxifene | 0.5 mg O |
| G04CA04 | Silodosin | 8 mg O |
| G04CX02 | Serenoa repens 6) | 0.32 g O |
| J01DI01 | Ceftobiprole medocaril | 1.5 g P |
| J01MA21 | Sitafloxacin | 0.1 g O |
| L03AC01 | Aldesleukin | 0.2 mg P |
| L03AX16 | Plerixafor | 16.8 mg P |
| L04AA04 | Antithymocyte immunoglobulin (rabbit) | 0.1 g P |
| L04AB06 | Golimumab | 1.66 mg P |
| L04AC07 | Tocilizumab | 20 mg P |
| L04AC08 | Canakinumab | 2.7 mg P |
| M05BB05 | Alendronic acid, calcium and colecalciferol, sequential | 10 mg3) O |
| M05BX04 | Denosumab | 0.33 mg P |
| N02AA55 | Oxycodone, combinations | 75 mg4) O |
| N02AB03 | Fentanyl | 0.6 mg N |
| N03AF04 | Eslicarbazepine | 0.8 g O |
| N05AH03 | Olanzapine | 10 mg P depot |
| N05AX13 | Paliperidone | 2.5 mg5) P depot |
| N06AX17 | Milnacipran | 0.1 g O |
| N06AX22 | Agomelatine | 25 mg O |
| N06BA12 | Lisdexamfetamine | 30 mg O |
| V03AE01 | Polystyrene sulfonate | 45 g O |
1) expressed as tocopherol
2) refers to fenofibric acid
3) refers to alendronic acid
4) The DDD for N02AA55 oxycodone, combinations applies to combinations of oxycodone
and naloxone, only.
5) expressed as paliperidone
6) Assessed and approved by regulatory authorities based on dossiers including efficacy,
safety, and quality data
(e.g. the well-established use procedure in EU).
Full details on current ATC coding and defined daily doses (DDDs) can be obtained from the DUSC Secretary, Department of Health, GPO Box 9848, Canberra ACT 2601, or direct from the coordinating body: the WHO Collaborating Centre for Drug Statistics Methodology, Norwegian Institute of Public Health, PO BOX 4404 Nydalen 0403 Oslo Norway, or at their website: www.whocc.no




