ROSIGLITAZONE MALEATE, tablet, 4 mg (base) and 8 mg Avandia; and ROSIGLITAZONE MALEATE with METFORMIN HYDROCHLORIDE, 2 mg (base) – 500 mg, 2 mg (base) – 1 g, 4 mg (base) – 500 mg and 4 mg (base) – 1 g, Avandamet , November 2007
Public summary document for ROSIGLITAZONE MALEATE, tablet, 4 mg (base) and 8 mg Avandia; and ROSIGLITAZONE MALEATE with METFORMIN HYDROCHLORIDE, 2 mg (base) – 500 mg, 2 mg (base) – 1 g, 4 mg (base) – 500 mg and 4 mg (base) – 1 g, Avandamet , November 2007
Page last updated: 14 March 2008
Public Summary Document
Product: ROSIGLITAZONE MALEATE, tablet, 4 mg (base) and 8 mg Avandia; and ROSIGLITAZONE
MALEATE with METFORMIN HYDROCHLORIDE, 2 mg (base) – 500 mg, 2 mg (base) – 1 g, 4 mg
(base) – 500 mg and 4 mg (base) – 1 g, Avandamet
Sponsor: GlaxoSmithKline Australia Pty Ltd
Date of PBAC Consideration: November 2007
1. Purpose of Application
The submission sought to extend the current Authority Required PBS listing of rosiglitazone to include use as mono-therapy in type 2 diabetes mellitus patients who are uncontrolled on metformin or who cannot take metformin, and as dual oral combination therapy with metformin in patients who are uncontrolled on metformin alone.
2. Background
Rosiglitazone was first recommended for PBS listing at the March 2001 PBAC meeting
after being previously considered by the PBAC three times. The recommendation was
for an Authority required listing for use in combination therapy with either metformin
or a sulfonylurea in patients with type 2 diabetes mellitus and in whom a combination
of metformin and a sulfonylurea is either contraindicated or not tolerated.
At its November 2004 meeting, the PBAC recommended extending the PBS listing for rosiglitazone
to include triple therapy in type 2 diabetic patients whose diabetes is uncontrolled
while taking maximally tolerated doses of metformin and a sulfonylurea.
At its March 2005 meeting, the PBAC recommended extending the PBS listing of rosiglitazone
to include dual therapy in combination with insulin, for patients with type 2 diabetes
whose blood glucose concentrations are inadequately controlled.
In July 2006, the PBAC recommended a fixed dose combination of rosiglitazone and metformin
(Avandamet) for PBS listing.
3. Registration Status
Rosiglitazone’s TGA registration commenced on 13 July 2000. Rosiglitazone is indicated for the treatment of Type 2 diabetes mellitus (non-insulin dependent diabetes mellitus). It may be used in the following circumstances in patients inadequately controlled by diet and exercise:
- as monotherapy,
- in dual combination therapy with metformin, sulfonylureas or insulin,
- in triple combination therapy with metformin and sulfonylureas, to improve glycaemic control in patients with Type 2 diabetes mellitus.
4. Listing Requested and PBAC’s View:
Rosiglitazone maleate
Authority Required (Streamlined)
Type 2 diabetes, alone or in combination with other diabetes therapies, in a patient
whose HbA1c is greater than 7% prior to initiation of a thiazolidinedione (glitazone),
despite treatment with metformin, or where metformin is contra-indicated or not tolerated.
Rosiglitazone maleate with metformin hydrochloride
Authority Required (Streamlined)
Type 2 diabetes, alone or in combination with other diabetes therapies, in a patient
whose HbA1c is greater than 7% prior to initiation of a thiazolidinedione (glitazone),
despite treatment with metformin.
Note:
Avandamet is not PBS-subsidised when used in combination with insulin.
The PBAC made no comments on the restriction wording at this consideration.
5. Clinical Place for the Proposed Therapy
Current treatment guidelines recommend a stepwise approach to managing hyperglycaemia in type 2 diabetes, beginning with lifestyle modification, then initiating and intensifying treatment with oral anti-diabetic agents and eventually, insulin, whenever glycaemic control cannot be achieved. The submission proposes that rosiglitazone treatment be moved forward to replace the sulfonylureas in the treatment algorithm of type 2 diabetes. Improving lifestyle factors (i.e. diet and exercise) followed by metformin therapy will remain as preferred treatment options before initiating rosiglitazone therapy.
6. Comparator
The submission appropriately nominated the group of sulfonylurea drugs that are currently PBS listed: glibenclamide, gliclazide, glimepiride and glipizide as the comparator.
7. Clinical Trials
For the monotherapy restriction, the submission presented the results of one key trial
(Trial 048) and three supportive trials (Trials 020, 080 and 097) comparing rosiglitazone
with sulfonylureas in patients with type 2 diabetes. For the dual therapy restriction,
the submission presented the results of two key trials (Trials 231 and 264) and two
supportive trials (Garber, 2006 and Derosa, 2005) comparing metformin plus rosiglitazone
with metformin plus sulfonylurea in patients with type 2 diabetes.
The trials published at the time of submission are as follows:
|
Trial/First author |
Protocol/Publication title |
Publication citation |
|---|---|---|
|
Direct randomised trials for monotherapy indication |
||
|
Trial 048/ Kahn SE |
A randomized, double-blind study to compare the durability of glucose lowering and preservation of pancreatic beta-cell function of rosiglitazone monotherapy compared to metformin or glyburide/glibenclamide in subjects with drug-naive recently diagnosed type 2 diabetes mellitus. |
N Engl J Med 2006; 355:2427-2443. |
|
Trial 020/ |
A multicentre, double-blind, parallel group comparative study to evaluate the efficacy, safety and tolerability of rosiglitazone vs. glibenclamide therapy, when administered to patients with type 2 diabetes mellitus. |
(1) Nutr Metab Cardiovasc Dis 2007; 17:13-23. |
|
Trial 080/ |
A 3 year open-label, multicenter, active (glyburide) comparison study to evaluate the effect of rosiglitazone on cardiovascular function in patients with non-insulin dependent diabetes mellitus (NIDDM). |
(1) J Hum Hypertens 2003; 17:7-12. |
|
Direct randomised trials for dual therapy indication |
||
|
Trial 231/ |
RECORD: Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of glycaemia in Diabetes. A long term, open label, randomised study in patients with type 2 diabetes, comparing the combination of rosiglitazone and either metformin or sulphonylurea with metformin plus sulphonylurea on cardiovascular endpoints and glycaemia: an 18 month interim analysis of the efficacy of the combination of rosiglitazone and either metformin or sulphonylurea vs. metformin plus sulphonylurea. |
(1) Diabet Med 2007; 24:626-634. |
|
Garber et al 2006 |
Metformin-glibenclamide versus metformin plus rosiglitazone in patients with type 2 diabetes inadequately controlled on metformin monotherapy. |
Diabetes Obes Metab 2006; 8:156-163. |
|
Derosa et al 2005 |
Long-term effects of glimepiride or rosiglitazone in combination with metformin on blood pressure control in type 2 diabetic patients affected by the metabolic syndrome: a 12-month, double-blind, randomized clinical trial. |
Clin Ther 2005; 27:1383-1391. |
|
Derosa et al 2005 |
Antithrombotic effects of rosiglitazone-metformin versus glimepiride-metformin combination therapy in patients with type 2 diabetes mellitus and metabolic syndrome. |
Pharmacotherapy 2005; 25:637-645. |
|
Derosa et al 2005 |
Long-term effect of glimepiride and rosiglitazone on non-conventional cardiovascular risk factors in metformin-treated patients affected by metabolic syndrome: a randomized, double-blind clinical trial. |
J Int Med Res 2005; 33:284-294. |
8. Results of Trials
The results of the key trials are summarised in the tables below.
Time to monotherapy failure (FPG >180mg/dL): Monotherapy Trial 048
|
No. with event |
Cumulative incidence |
Hazard ratio |
Log rank p-value |
||
|---|---|---|---|---|---|
|
RSG |
SU |
RSG |
SU |
||
|
143/1393 |
311/1337 |
0.15 (0.12, 0.17) |
0.34 (0.30, 0.37) |
0.37 (0.30, 0.45) |
<0.0001 |
Rosiglitazone treatment significantly reduced the risk of monotherapy failure by 63%
relative to sulfonylurea treatment in Trial 048.
Mean change from baseline in HbA1c (%): Monotherapy trials
|
Trial ID |
Rosiglitazone[mean (SD)] |
Sulfonylurea[mean (SD)] |
Mean difference |
||
|---|---|---|---|---|---|
|
Baseline |
Change |
Baseline |
Change |
||
|
Trial 048 |
7.36 (0.93) |
-0.35 (0.03) |
7.36 (0.92) |
0.07 (0.03) |
-0.42 (-0.50, -0.33) |
|
Trial 020 |
8mg bd |
-0.53 (1.31) |
8.15 (1.26) |
-0.72 (1.00) |
0.21 (-0.01, 0.42) |
|
Trial 080 |
9.1 (1.7) |
-0.9 (1.4) |
9.5 (1.6) |
-0.9 (1.4) |
NR |
NR – not reported
In Trial 048 the reduction in HbA1c was statistically significantly greater in rosiglitazone-treated
patients than patients treated with sulfonylureas. In the unpublished trial 097, the
HbA1c decrease in the sulfonylurea group was significantly greater than the decrease
in the rosiglitazone group. There was no difference between treatment groups in Trials
020 and 080.
The submission claimed that while sulfonylureas produce an early, relatively short-lived
impact on HbA1c, rosiglitazone produces a more gradual and sustained impact on HbA1c.
Mean HbA1c (%) by visit to 48 months in trial 048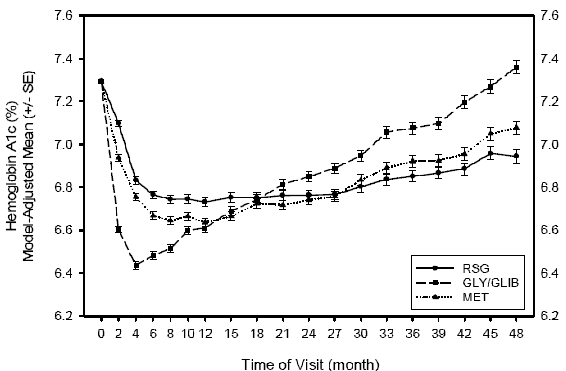
In the key dual therapy trials, patients treated with metformin plus sulfonylurea
had numerically greater reductions in HbA1c compared with patients treated with metformin
plus rosiglitazone, but the differences were not statistically significant.
Mean HbA1c (%) by visit to 18 months: Home et al (2007)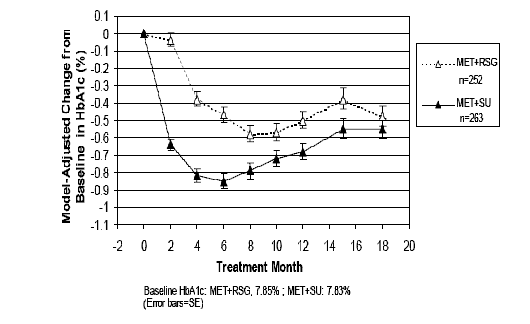
Mean HbA1c dropped more rapidly (and to a greater extent) in the metformin plus sulfonylurea
group than in the metformin plus rosiglitazone group. This trend suggests there may
be some HbA1c benefit to patients associated with metformin plus sulfonylurea compared
to metformin plus rosiglitazone.
The most common adverse events occurring in Trial 048 that were assessed as related
to the trial medication were hypoglycaemia (38.7% in sulfonylurea-treated patients
versus 9.8% in rosiglitazone-treated patients) and peripheral oedema and increased
weight (both of which occurred at higher rates in the rosiglitazone group compared
to the sulfonylurea group).
Trial 048 analysed pre-determined adverse events of special interest: the sulfonylurea
group had higher rates of hepatic adverse events (any events, events leading to withdrawal)
and hypoglycaemia (any events, serious events and events leading to withdrawal) compared
to the rosiglitazone group; and the rosiglitazone group had higher rates of serious
cardiovascular events, haematological events, anaemia, negative effects on LDL cholesterol
levels, weight gain, oedema and fractures (in females) compared to the sulfonylurea
group.
The submission also presented an extended assessment of comparative harms. This review
identified three meta-analyses (one by the sponsor, one by the US FDA and one independent
analysis) indicating that rosiglitazone may be associated with an increase in the
risk of myocardial infarction. This was of concern to the PBAC.
For the PBAC’s comments on these results, see Recommendation and Reasons.
9. Clinical Claim
The submission claimed that rosiglitazone offers superior efficacy over sulfonylureas with similar, but slightly uncertain, safety. The PBAC considered that the safety concerns may outweigh the incremental benefit of earlier rosiglitazone therapy, which in itself was uncertain (see Recommendation and Reasons).
10. Economic Analysis
Two modelled economic evaluations were presented in the submission, one for monotherapy,
and one for dual therapy.
Each model followed a cohort of 10,000 patients for 20 years through a treatment algorithm,
based on their simulated baseline HbA1c and the effect of their treatment on their
HbA1c. Based on their HbA1c and risk equations from the UKPDS, patients may have experienced
(microvascular or macrovascular) complications or may have died. Patients may also
have experienced treatment-related adverse events (CHF, fractures, dyslipidaemia,
oedema, hypoglycaemia), which were based on trial event rates. Utilities and costs
were assigned to the treatment/complication health states. The resources included
were drug costs, the costs of treating adverse events (drugs, radiography, ED visits,
outpatient visits) and the costs of treating complications (GP visits, outpatient
visits, ambulance transport, ED visits and hospitalisation).
A base case modelled incremental discounted cost/QALY gained of less than $15,000
was calculated by the submission for the requested monotherapy restriction. For the
requested dual therapy restriction it was in the range $15,000 - $45,000.
The PBAC had serious doubts as to whether the difference in glycaemic control between
rosiglitazone and sulfonylureas is clinically important and whether the adverse events
associated with rosiglitazone do not outweigh any benefits, and thus considered the
economic analyses to be of questionable validity (see Recommendation and Reasons).
11. Estimated PBS Usage and Financial Implications
12. Recommendation and Reasons
The PBAC noted the expert at the hearing considered that safety problems with rosiglitazone
may have been over estimated. Further, if rosiglitazone were to be used earlier in
the treatment algorithm of type 2 diabetes in healthier patients, there may be fewer
concerns. Furthermore, earlier use in the treatment algorithm, when beta cells are
functioning more efficiently, would likely lead to better patient outcomes in both
macrovascular and microvascular endpoints and delayed use of insulin. The PBAC noted
that the expert did not consider the long term sequelae (if any) of the adverse effects
of the drug on increased fracture rates in females with earlier introduction of rosiglitazone.
In Trial 048, it was noted that the reduction in HbA1c at 48 months was statistically
significantly greater in rosiglitazone-treated patients than patients treated with
sulfonylureas. The PBAC noted that a number of recently published non-inferiority
trials in type 2 diabetes have used pre-defined non-inferiority margins of 0.4%-0.5%,
suggesting that the mean difference in HbA1c seen in the key Trial 048 after 48 months
of treatment (-0.42%) may not be clinically important. However, the PBAC noted that
the pre-PBAC response cited the 2002 American College of Endocrinology which stated
that any reduction in HbA1c is clinically important. The submission claimed that while
sulfonylureas produce an early, relatively short-lived impact on HbA1c, rosiglitazone
produces a more gradual and sustained impact on HbA1c. Although rosiglitazone produced
a more sustained impact on HbA1c, this effect also waned over time and the PBAC thus
considered that any net improvement was relatively small.
The PBAC considered it unlikely that the magnitude of the trial-reported gains in
reductions in HbA1c at 48 months with rosiglitazone would be realised in clinical
practice. Clinicians are likely to respond to the rebound of HbA1c over time in sulfonylurea-treated
patients by instituting dual therapy once HbA1c levels reached 7.0%. The PBAC noted
that the economic evaluation relied on the claim that rosiglitazone is more successful
than sulfonylureas in maintaining glycaemic control over time (i.e. the pattern of
the treatment effect). Despite the claims in the Pre-PBAC Response that this issue
had been addressed by the pre-modelling study in the submission, it remained as an
issue of uncertainty.
Estimates of reductions in cardiovascular outcomes with reductions in HbA1c were derived
from UKPDS risk equations. The PBAC noted the Pre-PBAC Response included a sensitivity
analysis which substituted Australian mortality rates for those observed in the UKPDS
and another which assumed no differences in deaths and cardiovascular outcomes or
strokes between treatment groups. However, the PBAC considered that there was considerable
uncertainty about whether the gains from HbA1c reduction predicted by UKPDS data are
applicable in the case of rosiglitazone because of rosiglitazone’s adverse events
profile. UKPDS data maps HbA1c changes to clinical endpoints based on metformin trial
data. The possible increased risk of adverse cardiovascular events with rosiglitazone
suggests that the extrapolations of cardiovascular benefits may not be appropriate
for this drug.
From the results of the key dual therapy trials, patients treated with metformin plus
sulfonylurea had numerically greater reductions in HbA1c compared with patients treated
with metformin plus rosiglitazone, but the differences were not statistically significant.
The mean HbA1c dropped more rapidly (and to a greater extent) in the metformin plus
sulfonylurea group than in the metformin plus rosiglitazone group, and there may thus
be some HbA1c benefit to patients associated with metformin plus sulfonylurea compared
to metformin plus rosiglitazone. Overall, the PBAC agreed that the clinical data provided
in the submission did not support the claim of greater benefits of metformin plus
rosiglitazone over dual therapy with metformin plus a sulfonylurea.
In terms of safety, the PBAC noted that rosiglitazone offers some benefit in terms
of fewer hypoglycaemic events, however rosiglitazone is associated with higher rates
of serious cardiovascular events, anaemia, negative effects on LDL cholesterol levels,
weight gain, oedema and fractures (in females) compared to sulfonylureas. Despite
the reassurances of the hearing, the PBAC considered there are serious concerns regarding
the safety of rosiglitazone, indicating that rosiglitazone may be associated with
an increase in the risk of myocardial infarction.
The TGA Advisor at the meeting confirmed that, currently, negotiations were underway
to include a boxed warning concerning the increased risk of myocardial ischaemia and
advising against use in patients with ischaemic heart disease. The PBAC noted that
in the published literature, up to 50% of type 2 diabetics have asymptomatic coronary
artery/myocardial disease on imaging, so there may potentially be an even greater
population than expected who would be at an additional cardiac risk by being on rosiglitazone.
In view of the larger number of patients likely to be exposed to rosiglitazone therapy
under the proposed restriction, the PBAC considered the considerable safety concerns
associated with rosiglitazone may outweigh the incremental benefit of earlier rosiglitazone
therapy, which in itself was uncertain, in terms of magnitude and duration of effect.
The choice of the cost-utility approach was considered to be of questionable validity
because of the small difference in glycaemic control between rosiglitazone and sulfonylureas
and the conclusion that the adverse events associated with earlier rosiglitazone may
outweigh any incremental benefits. Further, as stated above, the estimates are driven
by modelled reductions in cardiovascular events based on UKPDS risk equations which
may not be appropriate for rosiglitazone because the increased risk of some cardiovascular
events observed with rosiglitazone is not captured in the model’s application of HbA1c
changes to the UKPDS equations.
Other uncertainties relating to the model related to the utility estimates, which
the PBAC considered did not appear clinically plausible and are from a number of sources.
Also it is implausible that the disutility associated with hypoglycaemic events would
last for 6 months. Therefore, although hypoglycaemic events are an important outcome
with regard to morbidity and even mortality, especially in the elderly, the model
provided in the submission is not adequately informative on the benefits and risks
associated with rosiglitazone therapy.
The PBAC also noted that the utilisation and financial estimates have been overestimated.
The PBAC therefore rejected the submission because of considerable concern about the
safety of the drug, uncertain clinical benefit, and the resulting uncertain cost-effectiveness.
13. Context for Decision
The PBAC helps decide whether and, if so, how medicines should be subsidised in Australia. It considers submissions in this context. A PBAC decision not to recommend listing or not to recommend changing a listing does not represent a final PBAC view about the merits of the medicine. A company can resubmit to the PBAC or seek independent review of the PBAC decision.
14. Sponsor’s Comment
The sponsor has since consulted with the PBAC and Department of Health and Ageing to clarify the positioning and the valuation of the claimed benefits and will consider addressing these in a resubmission.



